Key Points
-
Modern organisms contain many protein-devoid RNA elements which have important roles in various cellular processes.
-
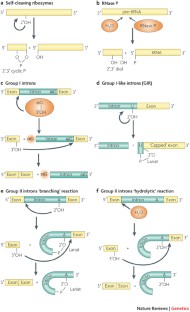
The collection of cleaving and splicing ribozymes has been recently supplemented by several eukaryotic and prokaryotic genome-encoded ribozymes that are present in pre-mRNA and mRNA molecules. These ribozymes seem to be involved in gene expression control and possibly in other basic cellular functions.
-
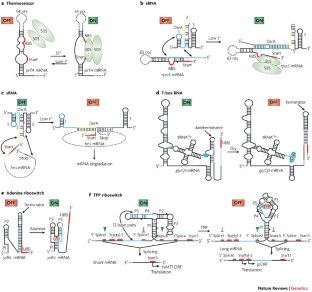
Other RNA-based elements and molecules, such as thermosensors, sRNAs, riboswitches and other RNA switches, can sense or react to changes in the environment and regulate gene expression through various mechanisms, including transcriptional, translational and splicing control.
-
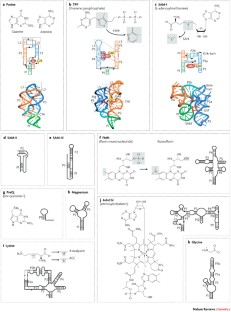
The three-dimensional structures of ribozymes and riboswitches share several architectural principals. Given this commonality, some of these RNA molecules show striking similarities in molecular recognition and mechanistic aspects of their function.
-
RNA-based elements are probably more versatile than previously thought: they show some features that are typical of proteins and, in some situations, they might have functional advantages over proteins. For example, riboswitches can directly sense the concentration of cellular metabolites and control gene expression without a requirement for proteins, conserving cellular resources and providing fine control of gene expression.
-
Although the origins and evolution of RNA-based elements are not clear, some of these RNAs might have descended from a hypothetical primordial 'RNA world'.
Abstract
Although various functions of RNA are carried out in conjunction with proteins, some catalytic RNAs, or ribozymes, which contribute to a range of cellular processes, require little or no assistance from proteins. Furthermore, the discovery of metabolite-sensing riboswitches and other types of RNA sensors has revealed RNA-based mechanisms that cells use to regulate gene expression in response to internal and external changes. Structural studies have shown how these RNAs can carry out a range of functions. In addition, the contribution of ribozymes and riboswitches to gene expression is being revealed as far more widespread than was previously appreciated. These findings have implications for understanding how cellular functions might have evolved from RNA-based origins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Cech, T. R., Zaug, A. J. & Grabowski, P. J. In vitro splicing of the ribosomal RNA precursor of Tetrahymena: involvement of a guanosine nucleotide in the excision of the intervening sequence. Cell 27, 487–496 (1981).
Guerrier-Takada, C., Gardiner, K., Marsh, T., Pace, N. & Altman, S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell 35, 849–857 (1983).
Gilbert, W. Origin of life: the RNA World. Nature 319, 618 (1986).
Mironov, A. S. et al. Sensing small molecules by nascent RNA: a mechanism to control transcription in bacteria. Cell 111, 747–756 (2002). References 4–6 describe the discovery of metabolite-binding riboswitches.
Nahvi, A. et al. Genetic control by a metabolite binding mRNA. Chem. Biol. 9, 1043–1049 (2002).
Winkler, W., Nahvi, A. & Breaker, R. R. Thiamine derivatives bind messenger RNAs directly to regulate bacterial gene expression. Nature 419, 952–956 (2002).
Winkler, W. C., Nahvi, A., Roth, A., Collins, J. A. & Breaker, R. R. Control of gene expression by a natural metabolite-responsive ribozyme. Nature 428, 281–286 (2004). This work showed that a riboswitch can function as a ribozyme.
Forster, A. C. & Symons, R. H. Self-cleavage of plus and minus RNAs of a virusoid and a structural model for the active sites. Cell 49, 211–220 (1987).
Buzayan, J. M., Gerlach, W. L. & Bruening, G. Non-enzymatic cleavage and ligation of RNAs complementary to a plant virus satellite RNA. Nature 323, 349–353 (1986).
Sharmeen, L., Kuo, M. Y., Dinter-Gottlieb, G. & Taylor, J. Antigenomic RNA of human hepatitis d virus can undergo self-cleavage. J. Virol. 62, 2674–2679 (1988).
Wu, H. N. et al. Human hepatitis δ virus RNA subfragments contain an autocleavage activity. Proc. Natl Acad. Sci USA 86, 1831–1835 (1989).
Saville, B. J. & Collins, R. A. A site-specific self-cleavage reaction performed by a novel RNA in Neurospora mitochondria. Cell 61, 685–696 (1990).
Salehi-Ashtiani, K., Luptak, A., Litovchick, A. & Szostak, J. W. A genomewide search for ribozymes reveals an HDV-like sequence in the human CPEB3 gene. Science 313, 1788–1792 (2006). The identification of several functional ribozymes in the human genome.
Teixeira, A. et al. Autocatalytic RNA cleavage in the human β-globin pre-mRNA promotes transcription termination. Nature 432, 526–530 (2004).
West, S., Gromak, N. & Proudfoot, N. J. Human 5′→3′ exonuclease XRN2 promotes transcription termination at co-transcriptional cleavage sites. Nature 432, 522–525 (2004).
Fedor, M. J. & Williamson, J. R. The catalytic diversity of RNAs. Nature Reviews Mol. Cell Biol. 6, 399–412 (2005).
Kikovska, E., Svard, S. G. & Kirsebom, L. A. Eukaryotic RNase P RNA mediates cleavage in the absence of protein. Proc. Natl Acad. Sci. USA 104, 2062–2067 (2007).
Chen, J. L. & Pace, N. R. Identification of the universally conserved core of ribonuclease P RNA. RNA 3, 557–560 (1997).
Lindahl, L., Fretz, S., Epps, N. & Zengel, J. M. Functional equivalence of hairpins in the RNA subunits of RNase MRP and RNase P in Saccharomyces cerevisiae. RNA 6, 653–658 (2000).
Michel, F. & Dujon, B. Conservation of RNA secondary structures in two intron families including mitochondrial-, chloroplast- and nuclear-encoded members. EMBO J. 2, 33–38 (1983).
Michel, F., Umesono, K. & Ozeki, H. Comparative and functional anatomy of group II catalytic introns — a review. Gene 82, 5–30 (1989).
Pyle, A. M. & Lambowitz, A. M. in The RNA World (eds Gesteland, R. F., Cech, T. R. & Atkins, J. F.) 469–506 (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, 2006).
Vogel, J. & Borner, T. Lariat formation and a hydrolytic pathway in plant chloroplast group II intron splicing. EMBO J. 21, 3794–3803 (2002).
Muller, M. W., Stocker, P., Hetzer, M. & Schweyen, R. J. Fate of the junction phosphate in alternating forward and reverse self-splicing reactions of group II intron RNA. J. Mol. Biol. 222, 145–154 (1991).
Nielsen, H., Westhof, E. & Johansen, S. An mRNA is capped by a 2′, 5′ lariat catalyzed by a group I-like ribozyme. Science 309, 1584–1587 (2005).
Altuvia, S., Kornitzer, D., Teff, D. & Oppenheim, A. B. Alternative mRNA structures of the cIII gene of bacteriophage λ determine the rate of its translation initiation. J. Mol. Biol. 210, 265–280 (1989).
Morita, M. T. et al. Translational induction of heat shock transcription factor sigma32: evidence for a built-in RNA thermosensor. Genes Dev. 13, 655–665 (1999).
Johansson, J. et al. An RNA thermosensor controls expression of virulence genes in Listeria monocytogenes. Cell 110, 551–561 (2002).
Chowdhury, S., Maris, C., Allain, F. H. & Narberhaus, F. Molecular basis for temperature sensing by an RNA thermometer. EMBO J. 25, 2487–2497 (2006).
Lybecker, M. C. & Samuels, D. S. Temperature-induced regulation of RpoS by a small RNA in Borrelia burgdorferi. Mol. Microbiol. 64, 1075–1089 (2007).
Shamovsky, I., Ivannikov, M., Kandel, E. S., Gershon, D. & Nudler, E. RNA-mediated response to heat shock in mammalian cells. Nature 440, 556–560 (2006).
Kolb, F. A. et al. Progression of a loop–loop complex to a four-way junction is crucial for the activity of a regulatory antisense RNA. EMBO J. 19, 5905–5915 (2000).
Lease, R. A., Cusick, M. E. & Belfort, M. Riboregulation in Escherichia coli: DsrA RNA acts by RNA:RNA interactions at multiple loci. Proc. Natl Acad. Sci. USA 95, 12456–12461 (1998).
Majdalani, N., Cunning, C., Sledjeski, D., Elliott, T. & Gottesman, S. DsrA RNA regulates translation of RpoS message by an anti-antisense mechanism, independent of its action as an antisilencer of transcription. Proc. Natl Acad. Sci. USA 95, 12462–12467 (1998).
Altuvia, S., Weinstein-Fischer, D., Zhang, A., Postow, L. & Storz, G. A small, stable RNA induced by oxidative stress: role as a pleiotropic regulator and antimutator. Cell 90, 43–53 (1997).
Altuvia, S., Zhang, A., Argaman, L., Tiwari, A. & Storz, G. The Escherichia coli OxyS regulatory RNA represses fhlA translation by blocking ribosome binding. EMBO J. 17, 6069–6075 (1998).
Zhang, A. et al. The OxyS regulatory RNA represses rpoS translation and binds the Hfq (HF-I) protein. EMBO J. 17, 6061–6068 (1998).
Zhang, A., Wassarman, K. M., Ortega, J., Steven, A. C. & Storz, G. The Sm-like Hfq protein increases OxyS RNA interaction with target mRNAs. Mol. Cell 9, 11–22 (2002).
Grundy, F. J. & Henkin, T. M. tRNA as a positive regulator of transcription antitermination in B. subtilis. Cell 74, 475–482 (1993).
Grundy, F. J., Winkler, W. C. & Henkin, T. M. tRNA-mediated transcription antitermination in vitro: codon–anticodon pairing independent of the ribosome. Proc. Natl Acad. Sci. USA 99, 11121–11126 (2002).
Gagnon, Y. et al. Clustering and co-transcription of the Bacillus subtilis genes encoding the aminoacyl-tRNA synthetases specific for glutamate and for cysteine and the first enzyme for cysteine biosynthesis. J. Biol. Chem. 269, 7473–7482 (1994).
Breaker, R. R. in The RNA World (eds Gesteland, R. F., Cech, T. R. & Atkins, J. F.) 89–108 (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, 2006).
Cheah, M. T., Wachter, A., Sudarsan, N. & Breaker, R. R. Control of alternative RNA splicing and gene expression by eukaryotic riboswitches. Nature 447, 391–393 (2007). References 43 and 44 established the involvement of eukaryotic riboswitches in the control of alternative splicing.
Kubodera, T. et al. Thiamine-regulated gene expression of Aspergillus oryzae thiA requires splicing of the intron containing a riboswitch-like domain in the 5′-UTR. FEBS Lett. 555, 516–520 (2003).
Mandal, M. & Breaker, R. R. Adenine riboswitches and gene activation by disruption of a transcription terminator. Nature Struct. Mol. Biol. 11, 29–35 (2004).
Corbino, K. A. et al. Evidence for a second class of S-adenosylmethionine riboswitches and other regulatory RNA motifs in α-proteobacteria. Genome Biol. 6, R70 (2005).
Epshtein, V., Mironov, A. S. & Nudler, E. The riboswitch-mediated control of sulfur metabolism in bacteria. Proc. Natl Acad. Sci. USA 100, 5052–5056 (2003).
Fuchs, R. T., Grundy, F. J. & Henkin, T. M. The SMK box is a new SAM-binding RNA for translational regulation of SAM synthetase. Nature Struct. Mol. Biol. 13, 226–233 (2006).
McDaniel, B. A., Grundy, F. J., Artsimovitch, I. & Henkin, T. M. Transcription termination control of the S box system: direct measurement of S-adenosylmethionine by the leader RNA. Proc. Natl Acad. Sci. USA 100, 3083–3088 (2003).
Winkler, W. C., Cohen-Chalamish, S. & Breaker, R. R. An mRNA structure that controls gene expression by binding FMN. Proc. Natl Acad. Sci. USA 99, 15908–15913 (2002).
Nou, X. & Kadner, R. J. Adenosylcobalamin inhibits ribosome binding to btuB RNA. Proc. Natl Acad. Sci. USA 97, 7190–7195 (2000).
Winkler, W. C., Nahvi, A., Sudarsan, N., Barrick, J. E. & Breaker, R. R. An mRNA structure that controls gene expression by binding S-adenosylmethionine. Nature Struct. Biol. 10, 701–707 (2003).
Mandal, M. et al. A glycine-dependent riboswitch that uses cooperative binding to control gene expression. Science 306, 275–279 (2004).
Sudarsan, N., Wickiser, J. K., Nakamura, S., Ebert, M. S. & Breaker, R. R. An mRNA structure in bacteria that controls gene expression by binding lysine. Genes Dev. 17, 2688–2697 (2003).
Mandal, M., Boese, B., Barrick, J. E., Winkler, W. C. & Breaker, R. R. Riboswitches control fundamental biochemical pathways in Bacillus subtilis and other bacteria. Cell 113, 577–586 (2003).
Roth, A. et al. A riboswitch selective for the queuosine precursor preQ1 contains an unusually small aptamer domain. Nature Struct. Mol. Biol. 14, 308–317 (2007).
Cromie, M. J., Shi, Y., Latifi, T. & Groisman, E. A. An RNA sensor for intracellular Mg2+. Cell 125, 71–84 (2006).
Barrick, J. E. et al. New RNA motifs suggest an expanded scope for riboswitches in bacterial genetic control. Proc. Natl Acad. Sci. USA 101, 6421–6426 (2004).
Sudarsan, N., Cohen-Chalamish, S., Nakamura, S., Emilsson, G. M. & Breaker, R. R. Thiamine pyrophosphate riboswitches are targets for the antimicrobial compound pyrithiamine. Chem. Biol. 12, 1325–1335 (2005).
Blount, K. F., Wang, J. X., Lim, J., Sudarsan, N. & Breaker, R. R. Antibacterial lysine analogs that target lysine riboswitches. Nature Chem. Biol. 3, 44–49 (2007).
Blount, K. F. & Breaker, R. R. Riboswitches as antibacterial drug targets. Nature Biotechnol. 24, 1558–1564 (2006).
Batey, R. T., Gilbert, S. D. & Montange, R. K. Structure of a natural guanine-responsive riboswitch complexed with the metabolite hypoxanthine. Nature 432, 411–415 (2004). References 62 and 66 describe the first three-dimensional structures of riboswitches.
Edwards, T. E. & Ferre-D'Amare, A. R. Crystal structures of the thi-box riboswitch bound to thiamine pyrophosphate analogs reveal adaptive RNA–small molecule recognition. Structure 14, 1459–1468 (2006).
Montange, R. K. & Batey, R. T. Structure of the S-adenosylmethionine riboswitch regulatory mRNA element. Nature 441, 1172–1175 (2006).
Serganov, A., Polonskaia, A., Phan, A. T., Breaker, R. R. & Patel, D. J. Structural basis for gene regulation by a thiamine pyrophosphate-sensing riboswitch. Nature 441, 1167–1171 (2006).
Serganov, A. et al. Structural basis for discriminative regulation of gene expression by adenine- and guanine-sensing mRNAs. Chem. Biol. 11, 1729–1741 (2004).
Thore, S., Leibundgut, M. & Ban, N. Structure of the eukaryotic thiamine pyrophosphate riboswitch with its regulatory ligand. Science 312, 1208–1211 (2006).
Adams, P. L., Stahley, M. R., Kosek, A. B., Wang, J. & Strobel, S. A. Crystal structure of a self-splicing group I intron with both exons. Nature 430, 45–50 (2004). References 68, 71, 72 and 79 provide insights into the group I intron self-splicing mechanism at the atomic level.
Cochrane, J. C., Lipchock, S. V. & Strobel, S. A. Structural investigation of the GlmS ribozyme bound to its catalytic cofactor. Chem. Biol. 14, 97–105 (2007). References 69 and 75 describe the crystal structures of the glmS ribozyme.
Ferre-D'Amare, A. R., Zhou, K. & Doudna, J. A. Crystal structure of a hepatitis δ virus ribozyme. Nature 395, 567–574 (1998).
Golden, B. L., Kim, H. & Chase, E. Crystal structure of a phage Twort group I ribozyme-product complex. Nature Struct. Mol. Biol. 12, 82–89 (2005).
Guo, F., Gooding, A. R. & Cech, T. R. Structure of the Tetrahymena ribozyme: base triple sandwich and metal ion at the active site. Mol. Cell 16, 351–362 (2004).
Kazantsev, A. V. et al. Crystal structure of a bacterial ribonuclease P RNA. Proc. Natl Acad. Sci. USA 102, 13392–13397 (2005).
Ke, A., Zhou, K., Ding, F., Cate, J. H. & Doudna, J. A. A conformational switch controls hepatitis d virus ribozyme catalysis. Nature 429, 201–205 (2004).
Klein, D. J. & Ferre-D'Amare, A. R. Structural basis of glmS ribozyme activation by glucosamine-6-phosphate. Science 313, 1752–1756 (2006).
Martick, M. & Scott, W. G. Tertiary contacts distant from the active site prime a ribozyme for catalysis. Cell 126, 309–320 (2006).
Rupert, P. B. & Ferre-D'Amare, A. R. Crystal structure of a hairpin ribozyme-inhibitor complex with implications for catalysis. Nature 410, 780–786 (2001).
Rupert, P. B., Massey, A. P., Sigurdsson, S. T. & Ferre-D'Amare, A. R. Transition state stabilization by a catalytic RNA. Science 298, 1421–1424 (2002).
Stahley, M. R. & Strobel, S. A. Structural evidence for a two-metal-ion mechanism of group I intron splicing. Science 309, 1587–1590 (2005).
Torres-Larios, A., Swinger, K. K., Krasilnikov, A. S., Pan, T. & Mondragon, A. Crystal structure of the RNA component of bacterial ribonuclease P. Nature 437, 584–587 (2005).
Westhof, E., Masquida, B. & Jaeger, L. RNA tectonics: towards RNA design. Fold. Des. 1, R78–88 (1996).
Lescoute, A. & Westhof, E. Topology of three-way junctions in folded RNAs. RNA 12, 83–93 (2006).
De la Pena, M., Gago, S. & Flores, R. Peripheral regions of natural hammerhead ribozymes greatly increase their self-cleavage activity. EMBO J. 22, 5561–5570 (2003).
Khvorova, A., Lescoute, A., Westhof, E. & Jayasena, S. D. Sequence elements outside the hammerhead ribozyme catalytic core enable intracellular activity. Nature Struct. Biol. 10, 708–712 (2003).
Krasilnikov, A. S., Yang, X., Pan, T. & Mondragon, A. Crystal structure of the specificity domain of ribonuclease P. Nature 421, 760–764 (2003).
Waldsich, C. & Pyle, A. M. A folding control element for tertiary collapse of a group II intron ribozyme. Nature Struct. Mol. Biol. 14, 37–44 (2007).
Nudler, E. Flipping riboswitches. Cell 126, 19–22 (2006).
Wickiser, J. K., Winkler, W. C., Breaker, R. R. & Crothers, D. M. The speed of RNA transcription and metabolite binding kinetics operate an FMN riboswitch. Mol. Cell 18, 49–60 (2005).
Wickiser, J. K., Cheah, M. T., Breaker, R. R. & Crothers, D. M. The kinetics of ligand binding by an adenine-sensing riboswitch. Biochemistry 44, 13404–13414 (2005).
Semrad, K. & Schroeder, R. A ribosomal function is necessary for efficient splicing of the T4 phage thymidylate synthase intron in vivo. Genes Dev. 12, 1327–1337 (1998).
Ferat, J. L., Le Gouar, M. & Michel, F. A group II intron has invaded the genus Azotobacter and is inserted within the termination codon of the essential groEL gene. Mol. Microbiol. 49, 1407–1423 (2003).
Jenkins, K. P., Hong, L. & Hallick, R. B. Alternative splicing of the Euglena gracilis chloroplast roaA transcript. RNA 1, 624–633 (1995).
Vogel, J. & Hess, W. R. Complete 5′ and 3′ end maturation of group II intron-containing tRNA precursors. RNA 7, 285–292 (2001).
Przybilski, R. et al. Functional hammerhead ribozymes naturally encoded in the genome of Arabidopsis thaliana. Plant Cell 17, 1877–1885 (2005).
Ferbeyre, G., Bourdeau, V., Pageau, M., Miramontes, P. & Cedergren, R. Distribution of hammerhead and hammerhead-like RNA motifs through the GenBank. Genome Res. 10, 1011–1019 (2000).
Graf, S., Przybilski, R., Steger, G. & Hammann, C. A database search for hammerhead ribozyme motifs. Biochem. Soc. Trans. 33, 477–478 (2005).
Gill, T., Cai, T., Aulds, J., Wierzbicki, S. & Schmitt, M. E. RNase MRP cleaves the CLB2 mRNA to promote cell cycle progression: novel method of mRNA degradation. Mol. Cell. Biol. 24, 945–953 (2004).
Sudarsan, N. et al. Tandem riboswitch architectures exhibit complex gene control functions. Science 314, 300–304 (2006).
Rodionov, D. A., Dubchak, I., Arkin, A., Alm, E. & Gelfand, M. S. Reconstruction of regulatory and metabolic pathways in metal-reducing δ-proteobacteria. Genome Biol. 5, R90 (2004).
Putzer, H., Gendron, N. & Grunberg-Manago, M. Co-ordinate expression of the two threonyl-tRNA synthetase genes in Bacillus subtilis: control by transcriptional antitermination involving a conserved regulatory sequence. EMBO J. 11, 3117–3127 (1992).
Lease, R. A. & Belfort, M. A trans-acting RNA as a control switch in Escherichia coli: DsrA modulates function by forming alternative structures. Proc. Natl Acad. Sci. USA 97, 9919–9924 (2000).
Sudarsan, N., Barrick, J. E. & Breaker, R. R. Metabolite-binding RNA domains are present in the genes of eukaryotes. RNA 9, 644–647 (2003).
Griffiths-Jones, S. et al. Rfam: annotating non-coding RNAs in complete genomes. Nucleic Acids Res. 33, D121–D124 (2005).
Borsuk, P. et al. L-arginine influences the structure and function of arginase mRNA in Aspergillus nidulans. Biol. Chem. 388, 135–144 (2007).
Hertel, K. J., Peracchi, A., Uhlenbeck, O. C. & Herschlag, D. Use of intrinsic binding energy for catalysis by an RNA enzyme. Proc. Natl Acad. Sci. USA 94, 8497–8502 (1997).
Tanner, N. K. et al. A three-dimensional model of hepatitis δ virus ribozyme based on biochemical and mutational analyses. Curr. Biol. 4, 488–498 (1994).
Canny, M. D. et al. Fast cleavage kinetics of a natural hammerhead ribozyme. J. Amer. Chem. Soc. 126, 10848–10849 (2004).
Richards, F. M. & Wyckoff, H. W. in The Enzymes (ed. Boyer, P. D.) 647–806 (Academic, New York, 1971).
Nahas, M. K. et al. Observation of internal cleavage and ligation reactions of a ribozyme. Nature Struct. Mol. Biol. 11, 1107–1113 (2004).
Rodionov, D. A., Vitreschak, A. G., Mironov, A. A. & Gelfand, M. S. Comparative genomics of the methionine metabolism in Gram-positive bacteria: a variety of regulatory systems. Nucleic Acids Res. 32, 3340–3353 (2004).
Salehi-Ashtiani, K. & Szostak, J. W. In vitro evolution suggests multiple origins for the hammerhead ribozyme. Nature 414, 82–84 (2001).
Koonin, E. V. The origin of introns and their role in eukaryogenesis: a compromise solution to the introns-early versus introns-late debate? Biol. Direct 1, 22 (2006).
White, H. B. 3rd. Coenzymes as fossils of an earlier metabolic state. J. Mol. Evol. 7, 101–104 (1976).
Hammann, C. & Westhof, E. Searching genomes for ribozymes and riboswitches. Genome Biol. 8, 210 (2007).
Noeske, J. et al. An intermolecular base triple as the basis of ligand specificity and affinity in the guanine- and adenine-sensing riboswitch RNAs. Proc. Natl Acad. Sci. USA 102, 1372–1377 (2005).
Serganov, A. et al. Structural basis for Diels–Alder ribozyme-catalyzed carbon–carbon bond formation. Nature Struct. Mol. Biol. 12, 218–224 (2005).
Ke, A., Ding, F., Batchelor, J. D. & Doudna, J. A. Structural roles of monovalent cations in the HDV ribozyme. Structure 15, 281–287 (2007).
Beattie, T. L., Olive, J. E. & Collins, R. A. A secondary structure model for the self-cleaving region of Neurospora VS RNA. Proc. Natl Acad. Sci. USA 92, 4686–4690 (1995).
Torres-Larios, A., Swinger, K. K., Pan, T. & Mondragon, A. Structure of ribonuclease P — a universal ribozyme. Curr. Opin. Struct. Biol. 16, 327–335 (2006).
Woodson, S. A. Structure and assembly of group I introns. Curr. Opin. Struct. Biol. 15, 324–330 (2005).
Rolle, K., Zywicki, M., Wyszko, E., Barciszewska, M. Z. & Barciszewski, J. Evaluation of the dynamic structure of DsrA RNA from E. coli and its functional consequences. J. Biochem. (Tokyo) 139, 431–438 (2006).
Brown, L. & Elliott, T. Mutations that increase expression of the rpoS gene and decrease its dependence on hfq function in Salmonella typhimurium. J. Bacteriol. 179, 656–662 (1997).
Masse, E., Escorcia, F. E. & Gottesman, S. Coupled degradation of a small regulatory RNA and its mRNA targets in Escherichia coli. Genes Dev. 17, 2374–2383 (2003).
Yousef, M. R., Grundy, F. J. & Henkin, T. M. Structural transitions induced by the interaction between tRNAGly and the Bacillus subtilis glyQS T box leader RNA. J. Mol. Biol. 349, 273–287 (2005).
Yousef, M. R., Grundy, F. J. & Henkin, T. M. tRNA requirements for glyQS antitermination: a new twist on tRNA. RNA 9, 1148–1156 (2003).
Altuvia, S. & Wagner, E. G. H. Switching on and off with RNA. Proc. Natl Acad. Sci. USA 97, 9824–9826 (2000).
Acknowledgements
This work was supported by the US National Institutes of Health grant GM073618.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Protein Data Bank Identification (PDB ID)
Asoarcus spp. BH72 group I intron
Bacillus antracis glmS ribozyme
Bacillus subtilis RNase P, B-type
Thermoanaerobacter tengcongensis riboswitch
FURTHER INFORMATION
Glossary
- Satellite RNAs
-
Subviral agents whose multiplication in a host cell depends on coinfection with a helper virus.
- Rolling-circle replication
-
A process of replication of some circular genomes, whereby one strand is replicated first and the second strand is replicated after completion of the first one.
- RNA maturase
-
A protein enzyme, intron-specific, that acts as a cofactor to facilitate splicing.
- Homing endonucleases
-
dsDNA-specific deoxyribonucleases that recognize large asymmetrical DNA sequences, which are not stringently defined, and which mobilize these DNA elements by facilitating their integration into new genomic sites. Because these sites lack introns and inteins (protein introns), this form of mobility is termed 'homing'. Homing endonucleases are encoded by intervening sequences embedded in either introns or inteins.
- Reverse transcriptase
-
An RNA-dependent DNA polymerase that transcribes ssRNA into dsDNA.
- Transcriptional attenuation
-
A regulatory mechanism whereby gene expression is controlled through the formation of alternative structures in the mRNA sequence that inhibit or facilitate the progression of transcription.
- Aptamer
-
Derived from the Latin aptus, meaning 'to fit', aptamers are oligonucleotide or peptide molecules that bind specific target molecules. The term is usually applied to nucleic acid molecules that are created following selection from a large random sequence pool, as well as to natural metabolite-sensing domains in riboswitches, which possess similar recognition properties to artificially generated aptamers.
- Boolean NOR logic gate
-
Boolean logic, named after George Boole, is a complete system for logical operations. A logic gate performs a logical operation on one or more logical inputs and produces a single logical output. The logical NOR, or joint denial, is a logical operator, meaning that the output is true if none of the inputs are true. Consequently, if one or both inputs are true, then the output is not true.
- Prosthetic group
-
A non-protein component of a conjugated protein that is usually essential for the protein's function. Prosthetic groups are also called coenzymes and cofactors.
- Lateral transfer
-
A process of transferring genetic material from one organism to another without reproduction. Also called horizontal gene transfer.
- Telomerase
-
An RNA-containing reverse transcriptase that adds DNA sequence repeats (telomeric repeats) to the 3′ end of DNA strands in eukaryotic chromosomes using its RNA component as a synthesis template.
Rights and permissions
About this article
Cite this article
Serganov, A., Patel, D. Ribozymes, riboswitches and beyond: regulation of gene expression without proteins. Nat Rev Genet 8, 776–790 (2007). https://doi.org/10.1038/nrg2172
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg2172
This article is cited by
-
Synthesis of [7-15N]-GTPs for RNA structure and dynamics by NMR spectroscopy
Monatshefte für Chemie - Chemical Monthly (2022)
-
Dynamics of transcription–translation coordination tune bacterial indole signaling
Nature Chemical Biology (2020)
-
NADP modulates RNA m6A methylation and adipogenesis via enhancing FTO activity
Nature Chemical Biology (2020)
-
Comprehensive sequence-to-function mapping of cofactor-dependent RNA catalysis in the glmS ribozyme
Nature Communications (2020)



