Key Points
-
Mutations in the maternally inherited mitochondrial genome (mtDNA), which contributes essential protein subunits to the enzyme complexes of oxidative phosphorylation, are an important cause of genetic disease.
-
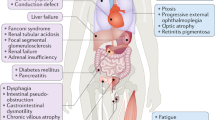
The clinical manifestations of mtDNA disorders are extremely variable; onset of symptoms might occur in infancy or in adulthood and can involve either a single organ or multiple tissues.
-
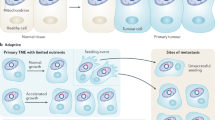
Multiple copies (several hundreds or thousands) of the mitochondrial genome are present in individual cells. Many pathogenic mutations only affect a subset of mtDNA molecules, and there are differences in the mutation load between tissues that contribute to the observed clinical heterogeneity.
-
Treatment options for mtDNA disorders are extremely limited, although several new approaches are being considered, including methods to prevent the transmission of pathogenic mutations from mother to offspring.
-
Somatic mtDNA mutations in both human tumours and tissues from ageing individuals are increasingly being described. Recent data from a mouse model engineered to lose mtDNA sequence integrity supports a causal link between mtDNA mutations and the ageing process.
Abstract
The human mitochondrial genome is extremely small compared with the nuclear genome, and mitochondrial genetics presents unique clinical and experimental challenges. Despite the diminutive size of the mitochondrial genome, mitochondrial DNA (mtDNA) mutations are an important cause of inherited disease. Recent years have witnessed considerable progress in understanding basic mitochondrial genetics and the relationship between inherited mutations and disease phenotypes, and in identifying acquired mtDNA mutations in both ageing and cancer. However, many challenges remain, including the prevention and treatment of these diseases. This review explores the advances that have been made and the areas in which future progress is likely.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Zeviani, M. & Di Donato, S. Mitochondrial disorders. Brain 127, 2153–2172 (2004).
Ivanov, P. L. et al. Mitochondrial DNA sequence heteroplasmy in the Grand Duke of Russia Georgij Romanov establishes the authenticity of the remains of Tsar Nicholas II. Nature Genet. 12, 417–420 (1996).
Anderson, S. et al. Sequence and organization of the human mitochondrial genome. Nature 290, 457–465 (1981). This paper describes the sequence and organization of human mtDNA — the first complete mitochondrial genome to be sequenced.
Bibb, M. J., Van Etten, R. A., Wright, C. T., Walberg, M. W. & Clayton, D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell 26, 167–180 (1981).
Anderson, S. et al. Complete sequence of bovine mitochondrial DNA. Conserved features of the mammalian mitochondrial genome. J. Mol. Biol. 156, 683–717 (1982).
Andrews, R. M. et al. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nature Genet. 23, 147 (1999). A re-analysis of the original human placental DNA sample used by Fred Sanger and colleagues, using automated, fluorescent DNA sequencing, which allowed a consensus human mtDNA sequence (the 'revised Cambridge Reference Sequence') to be made available.
Majamaa, K. et al. Epidemiology of A3243G, the mutation for mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes: prevalence of the mutation in an adult population. Am. J. Hum. Genet. 63, 447–454 (1998).
Schaefer, A. M., Taylor, R. W., Turnbull, D. M. & Chinnery, P. F. The epidemiology of mitochondrial disorders — past, present and future. Biochim. Biophys. Acta 1659, 115–120 (2004).
Wilson, F. H. et al. A cluster of metabolic defects caused by mutation in a mitochondrial tRNA. Science 306, 1190–1194 (2004).
Trifunovic, A. et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429, 417–423 (2004). This report describes a knock-in mouse model that expresses a proof-reading-deficient version of PolgA, the catalytic subunit of mtDNA polymerase, and provides the first in vivo data in mammals to establish a causative link between mtDNA mutations and ageing phenotypes.
Muller-Hocker, J. Cytochrome c oxidase deficient fibres in the limb muscle and diaphragm of man without muscular disease: an age-related alteration. J. Neurol. Sci. 100, 14–21 (1990).
Mootha, V. K. et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nature Genet. 34, 267–273 (2003).
Petersen, K. F. et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300, 1140–1142 (2003).
DiMauro, S. & Schon, E. A. Mitochondrial respiratory-chain diseases. N. Engl. J. Med. 348, 2656–2668 (2003).
Shoubridge, E. A. Nuclear genetic defects of oxidative phosphorylation. Hum. Mol. Genet. 10, 2277–2284 (2001).
Lang, B. F., Gray, M. W. & Burger, G. Mitochondrial genome evolution and the origin of eukaryotes. Annu. Rev. Genet. 33, 351–397 (1999).
Korhonen, J. A., Pham, X. H., Pellegrini, M. & Falkenberg, M. Reconstitution of a minimal mtDNA replisome in vitro. EMBO J. 23, 2423–2429 (2004).
Carrodeguas, J. A., Theis, K., Bogenhagen, D. F. & Kisker, C. Crystal structure and deletion analysis show that the accessory subunit of mammalian DNA polymerase-γ, Pol-γB, functions as a homodimer. Mol. Cell 7, 43–54 (2001).
Spelbrink, J. N. et al. Human mitochondrial DNA deletions associated with mutations in the gene encoding Twinkle, a phage T7 gene 4-like protein localized in mitochondria. Nature Genet. 28, 223–231 (2001).
Clayton, D. A. Replication of animal mitochondrial DNA. Cell 28, 693–705 (1982). This paper outlines the strand-displacement model of mammalian mtDNA replication, in which the two strands of mtDNA are each replicated in a continuous fashion from widely separated origins, requiring extensive displacement of parental DNA strands during leading-strand synthesis.
Holt, I. J., Lorimer, H. E. & Jacobs, H. T. Coupled leading- and lagging-strand synthesis of mammalian mitochondrial DNA. Cell 100, 515–524 (2000). Using two-dimensional agarose gel electrophoresis, the demonstration of duplex replication intermediates led the authors to propose a further mechanism for mammalian mtDNA replication that involves strand-coupled synthesis.
Yang, M. Y. et al. Biased incorporation of ribonucleotides on the mitochondrial L-strand accounts for apparent strand-asymmetric DNA replication. Cell 111, 495–505 (2002).
Bowmaker, M. et al. Mammalian mitochondrial DNA replicates bidirectionally from an initiation zone. J. Biol. Chem. 278, 50961–50969 (2003).
Bogenhagen, D. F. & Clayton, D. A. The mitochondrial DNA replication bubble has not burst. Trends Biochem. Sci. 28, 357–360 (2003).
Holt, I. J. & Jacobs, H. T. Response: The mitochondrial DNA replication bubble has not burst. Trends Biochem. Sci. 28, 355–356 (2003).
Bogenhagen, D. F. & Clayton, D. A. Concluding remarks: The mitochondrial DNA replication bubble has not burst. Trends Biochem. Sci. 28, 404–405 (2003).
Fish, J., Raule, N. & Attardi, G. Discovery of a major D-loop replication origin reveals two modes of human mtDNA synthesis. Science 306, 2098–2101 (2004).
Gaspari, M., Larsson, N. G. & Gustafsson, C. M. The transcription machinery in mammalian mitochondria. Biochim. Biophys. Acta 1659, 148–152 (2004).
Clayton, D. A. Replication and transcription of vertebrate mitochondrial DNA. Annu. Rev. Cell Biol. 7, 453–478 (1991).
Ojala, D., Montoya, J. & Attardi, G. tRNA punctuation model of RNA processing in human mitochondria. Nature 290, 470–474 (1981).
Falkenberg, M. et al. Mitochondrial transcription factors B1 and B2 activate transcription of human mtDNA. Nature Genet. 31, 289–294 (2002).
Fernandez-Silva, P., Enriquez, J. A. & Montoya, J. Replication and transcription of mammalian mitochondrial DNA. Exp. Physiol. 88, 41–56 (2003).
Gaspari, M., Falkenberg, M., Larsson, N. G. & Gustafsson, C. M. The mitochondrial RNA polymerase contributes critically to promoter specificity in mammalian cells. EMBO J. 23, 4606–4614 (2004).
Jacobs, H. T. Disorders of mitochondrial protein synthesis. Hum. Mol. Genet. 12, R293–R301 (2003).
Miller, C. et al. Defective mitochondrial translation caused by a ribosomal protein (MRPS16) mutation. Ann. Neurol. 56, 734–738 (2004).
Coenen, M. J. et al. Mutant mitochondrial elongation factor G1 and combined oxidative phosphorylation deficiency. N. Engl. J. Med. 351, 2080–2086 (2004).
Sciacco, M., Bonilla, E., Schon, E. A., DiMauro, S. & Moraes, C. T. Distribution of wild-type and common deletion forms of mtDNA in normal and respiration-deficient muscle fibers from patients with mitochondrial myopathy. Hum. Mol. Genet. 3, 13–19 (1994). A study of individual muscle fibres from patients with mitochondrial myopathy caused by large-scale mtDNA rearrangements revealed that, in addition to levels of deleted mtDNA that are above a critical threshold, respiratory-deficient fibres exhibit a marked reduction in the absolute amount of wild-type mtDNA.
Coller, H. A. et al. High frequency of homoplasmic mitochondrial DNA mutations in human tumors can be explained without selection. Nature Genet. 28, 147–150 (2001).
Taylor, R. W. et al. Mitochondrial DNA mutations in human colonic crypt stem cells. J. Clin. Invest. 112, 1351–1360 (2003).
Giles, R. E., Blanc, H., Cann, H. M. & Wallace, D. C. Maternal inheritance of human mitochondrial DNA. Proc. Natl Acad. Sci. USA 77, 6715–6719 (1980).
Gyllensten, U., Wharton, D., Josefsson, A. & Wilson, A. C. Paternal inheritance of mitochondrial DNA in mice. Nature 352, 255–257 (1991).
Shitara, H., Hayashi, J. I., Takahama, S., Kaneda, H. & Yonekawa, H. Maternal inheritance of mouse mtDNA in interspecific hybrids: segregation of the leaked paternal mtDNA followed by the prevention of subsequent paternal leakage. Genetics 148, 851–857 (1998).
Awadalla, P., Eyre-Walker, A. & Smith, J. M. Linkage disequilibrium and recombination in hominid mitochondrial DNA. Science 286, 2524–2525 (1999).
D'Aurelio, M. et al. Heterologous mitochondrial DNA recombination in human cells. Hum. Mol. Genet. 13, 3171–3179 (2004).
Kraytsberg, Y. et al. Recombination of human mitochondrial DNA. Science 304, 981 (2004).
Elson, J. L. et al. Analysis of European mtDNAs for recombination. Am. J. Hum. Genet. 68, 145–153 (2001).
Schwartz, M. & Vissing, J. Paternal inheritance of mitochondrial DNA. N. Engl. J. Med. 347, 576–580 (2002).
Taylor, R. W. et al. Genotypes from patients indicate no paternal mitochondrial DNA contribution. Ann. Neurol. 54, 521–524 (2003).
Filosto, M. et al. Lack of paternal inheritance of muscle mitochondrial DNA in sporadic mitochondrial myopathies. Ann. Neurol. 54, 524–526 (2003).
Schwartz, M. & Vissing, J. No evidence for paternal inheritance of mtDNA in patients with sporadic mtDNA mutations. J. Neurol. Sci. 218, 99–101 (2004)
Danan, C. et al. Evaluation of parental mitochondrial inheritance in neonates born after intracytoplasmic sperm injection. Am. J. Hum. Genet. 65, 463–473 (1999).
Marchington, D. R. et al. No evidence for paternal mtDNA transmission to offspring or extra-embryonic tissues after ICSI. Mol. Hum. Reprod. 8, 1046–1049 (2002).
Chinnery, P. F. et al. Risk of developing a mitochondrial DNA deletion disorder. Lancet 364, 592–596 (2004).
Man, P. Y. et al. The epidemiology of Leber hereditary optic neuropathy in the North East of England. Am. J. Hum. Genet. 72, 333–339 (2003).
Prezant, T. R. et al. Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced and non-syndromic deafness. Nature Genet. 4, 289–294 (1993).
Battersby, B. J., Loredo-Osti, J. C. & Shoubridge, E. A. Nuclear genetic control of mitochondrial DNA segregation. Nature Genet. 33, 183–186 (2003).
Brown, D. T., Samuels, D. C., Michael, E. M., Turnbull, D. M. & Chinnery, P. F. Random genetic drift determines the level of mutant mtDNA in human primary oocytes. Am. J. Hum. Genet. 68, 533–536 (2000).
Poulton, J. & Turnbull, D. M. 74th ENMC international workshop: mitochondrial diseases 19–20 November 1999, Naarden, the Netherlands. Neuromuscul. Disord. 10, 460–462 (2000).
Taylor, R. W., Schaefer, A. M., Barron, M. J., McFarland, R. & Turnbull, D. M. The diagnosis of mitochondrial muscle disease. Neuromuscul. Disord. 14, 237–245 (2004).
McFarland, R., Elson, J. L., Taylor, R. W., Howell, N. & Turnbull, D. M. Assigning pathogenicity to mitochondrial tRNA mutations: when 'definitely maybe' is not good enough. Trends Genet. 20, 591–596 (2004).
Holt, I. J., Harding, A. E. & Morgan-Hughes, J. A. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature 331, 717–719 (1988). The first demonstration that heteroplasmic, large-scale rearrangements of the mitochondrial genome could cause human disease.
Wallace, D. C. et al. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science 242, 1427–1430 (1988). This paper describes the first example of a single-nucleotide change in the mitochondrial genome (11778G>A) as the cause of a maternally inherited, neurological disorder in multiple families.
Brandon, M. C. et al. MITOMAP: a human mitochondrial genome database — 2004 update. Nucleic Acids Res. 33, D611–D613 (2005).
Moraes, C. T. et al. Mitochondrial DNA deletions in progressive external ophthalmoplegia and Kearns–Sayre syndrome. N. Engl. J. Med. 320, 1293–1299 (1989).
Rotig, A., Cormier, V., Blanche, S., Bonnefont, J. -P. & Ledeist, F. Pearson's marrow pancreas syndrome. A multisystem mitochondrial disorder of infancy. J. Clin. Invest. 86, 1601–1608 (1990).
McFarland, R. et al. De novo mutations in the mitochondrial ND3 gene as a cause of infantile mitochondrial encephalopathy and complex I deficiency. Ann. Neurol. 55, 58–64 (2004).
de Vries, D. D., van Engelen, B. G., Gabreels, F. J., Ruitenbeek, W. & van Oost, B. A. A second missense mutation in the mitochondrial ATPase 6 gene in Leigh's syndrome. Ann. Neurol. 34, 410–412 (1993).
Andreu, A. L. et al. Exercise intolerance due to mutations in the cytochrome b gene of mitochondrial DNA. N. Engl. J. Med. 341, 1037–1044 (1999).
Lowell, B. B. & Shulman, G. I. Mitochondrial dysfunction and type 2 diabetes. Science 307, 384–387 (2005).
Maassen, J. A. et al. Mitochondrial diabetes: molecular mechanisms and clinical presentation. Diabetes 53 (Suppl. 1), 103–109 (2004).
Saker, P. J. et al. UKPDS 21: low prevalence of the mitochondrial transfer RNA gene (tRNALeu(UUR)) mutation at position 3243bp in UK Caucasian type 2 diabetic patients. Diabet. Med. 14, 42–45 (1997).
Ohkubo, K. et al. Mitochondrial gene mutations in the tRNALeu(UUR) region and diabetes: prevalence and clinical phenotypes in Japan. Clin. Chem. 47, 1641–1648 (2001).
Kearney, P. M. et al. Global burden of hypertension: analysis of worldwide data. Lancet 365, 217–223 (2005).
Chinnery, P. F. et al. The epidemiology of pathogenic mitochondrial DNA mutations. Ann. Neurol. 48, 188–193 (2000).
Choo-Kang, A. T. et al. Defining the importance of mitochondrial gene defects in maternally inherited diabetes by sequencing the entire mitochondrial genome. Diabetes 51, 2317–2320 (2002).
Wallace, D. C. Mitochondrial genetics: a paradigm for aging and degenerative diseases? Science 256, 628–632 (1992).
Herrnstadt, C. et al. Reduced-median-network analysis of complete mitochondrial DNA coding-region sequences for the major African, Asian, and European haplogroups. Am. J. Hum. Genet. 70, 1152–1171 (2002).
Khogali, S. S. et al. A common mitochondrial DNA variant associated with susceptibility to dilated cardiomyopathy in two different populations. Lancet 357, 1265–1267 (2001).
Poulton, J. et al. Type 2 diabetes is associated with a common mitochondrial variant: evidence from a population-based case-control study. Hum. Mol. Genet. 11, 1581–1583 (2002).
Torroni, A. et al. Classification of European mtDNAs from an analysis of three European populations. Genetics 144, 1835–1850 (1996).
Chagnon, P. et al. Phylogenetic analysis of the mitochondrial genome indicates significant differences between patients with Alzheimer disease and controls in a French-Canadian founder population. Am. J. Med. Genet. 85, 20–30 (1999).
van der Walt, J. M. et al. Analysis of European mitochondrial haplogroups with Alzheimer disease risk. Neurosci. Lett. 365, 28–32 (2004).
Ross, O. A. et al. mt4216C variant in linkage with the mtDNA TJ cluster may confer a susceptibility to mitochondrial dysfunction resulting in an increased risk of Parkinson's disease in the Irish. Exp. Gerontol. 38, 397–405 (2003).
van der Walt, J. M. et al. Mitochondrial polymorphisms significantly reduce the risk of Parkinson disease. Am. J. Hum. Genet. 72, 804–811 (2003).
Helgason, A., Yngvadóttir, B., Hrafnkelsson, B., Gulcher, J. & Stefánsson, K. An Icelandic example of the impact of population structure on association studies. Nature Genet. 37, 90–95 (2005).
King, M. P. & Attardi, G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science 246, 500–503 (1989). This paper highlights the technology that has made trans mitochondrial cybrids — which are generated by fusing human cell lines that lack mtDNA to enucleated cytoplasts from patients' cells that harbour mtDNA mutations and then growing them under selection — such an elegant cell culture system to study the bioenergetic and cellular consequences of pathogenic mtDNA mutations.
Chomyn, A. et al. MELAS mutation in mtDNA binding site for transcription termination factor causes defects in protein synthesis and in respiration but no change in levels of upstream and downstream mature transcripts. Proc. Natl Acad. Sci. USA 89, 4221–4225 (1992).
Hayashi, J. et al. Introduction of disease-related mitochondrial DNA deletions into HeLa cells lacking mitochondrial DNA results in mitochondrial dysfunction. Proc. Natl Acad. Sci. USA 88, 10614–10618 (1991).
Tiranti, V. et al. Maternally inherited hearing loss, ataxia and myoclonus associated with a novel point mutation in mitochondrial tRNASer(UCN) gene. Hum. Mol. Genet. 4, 1421–1427 (1995).
Taanman, J. W. et al. Molecular mechanisms in mitochondrial DNA depletion syndrome. Hum. Mol. Genet. 6, 935–942 (1997).
Dunbar, D. R., Moonie, P. A., Jacobs, H. T. & Holt, I. J. Different cellular backgrounds confer a marked advantage to either mutant or wild-type mitochondrial genomes. Proc. Natl Acad. Sci. USA 92, 6562–6566 (1995).
El Meziane, A. et al. A tRNA supressor mutation in human mitochondria. Nature Genet. 18, 350–353 (1998).
Jenuth, J., Peterson, A. C., Fu, K. & Shoubridge, E. A. Random genetic drift in the female germ line explains the rapid segregation of mammalian mitochondrial DNA. Nature Genet. 14, 146–151 (1996). By generating heteroplasmic mice for two (neutral) mtDNA genotypes, the authors demonstrated that random genetic drift in early oogenesis was the reason for the observed rapid segregation of mtDNA sequence variants that occurs between generations.
Jenuth, J. P., Peterson, A. C. & Shoubridge, E. A. Tissue-specific selection for different mtDNA genotypes in heteroplasmic mice. Nature Genet. 16, 93–95 (1997).
Marchington, D. R., Barlow, D. & Poulton, J. Transmitochondrial mice carrying resistance to chloramphenicol on mitochondrial DNA: developing the first mouse model of mitochondrial DNA disease. Nature Med. 5, 957–960 (1999).
Sligh, J. E. et al. Maternal germ-line transmission of mutant mtDNAs from embryonic stem cell-derived chimeric mice. Proc. Natl Acad. Sci. USA 97, 14461–14466 (2000).
Inoue, K. et al. Generation of mice with mitochondrial dysfunction by introducing mouse mtDNA carrying a deletion into zygotes. Nature Genet. 26, 176–181 (2000). By isolating mouse cybrid clones with high levels of a somatic mtDNA rearrangement and fusing these with fertilized mouse eggs, these authors generated the first mouse model of a pathogenic mtDNA mutation (mtDNA deletion or duplication), which was transmitted from mother to offspring.
Nakada, K. et al. Inter-mitochondrial complementation: mitochondria-specific system preventing mice from expression of disease phenotypes by mutant mtDNA. Nature Med. 7, 934–940 (2001).
Larsson, N. G. et al. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nature Genet. 18, 231–236 (1998). The first Tfam knockout mouse model that demonstrates a role for the TFAM nuclear protein in maintaining mtDNA copy number.
Silva, J. P. et al. Impaired insulin secretion and β-cell loss in tissue-specific knockout mice with mitochondrial diabetes. Nature Genet. 26, 336–340 (2000).
Sorensen, L. et al. Late-onset corticohippocampal neurodepletion attributable to catastrophic failure of oxidative phosphorylation in MILON mice. J. Neurosci. 21, 8082–8090 (2001).
Wredenberg, A. et al. Increased mitochondrial mass in mitochondrial myopathy mice. Proc. Natl Acad. Sci. USA 99, 15066–15071 (2002).
Wang, J. et al. Dilated cardiomyopathy and atrioventricular conduction blocks induced by heart-specific inactivation of mitochondrial DNA gene expression. Nature Genet. 21, 133–137 (1999).
Chinnery, P. F. & Bindoff, L. A. 116th ENMC international workshop: the treatment of mitochondrial disorders, 14th–16th March 2003, Naarden, The Netherlands. Neuromuscul. Disord. 13, 757–764 (2003).
Taivassalo, T. et al. Gene shifting: a novel therapy for mitochondrial myopathy. Hum. Mol. Genet. 8, 1047–1052 (1999).
Clark, K. M. et al. Reversal of a mitochondrial DNA defect in human skeletal muscle. Nature Genet. 16, 222–224 (1997).
Fu, K. et al. A novel heteroplasmic tRNAleu(CUN) mtDNA point mutation in a sporadic patient with mitochondrial encephalomyopathy segregates rapidly in skeletal muscle and suggests an approach to therapy. Hum. Mol. Genet. 5, 1835–1840 (1996).
Taivassalo, T. et al. Aerobic conditioning in patients with mitochondrial myopathies: physiological, biochemical, and genetic effects. Ann. Neurol. 50, 133–141 (2001).
Manfredi, G. et al. Rescue of a deficiency in ATP synthesis by transfer of MTATP6, a mitochondrial DNA-encoded gene, to the nucleus. Nature Genet. 30, 394–399 (2002).
Guy, J. et al. Rescue of a mitochondrial deficiency causing Leber Hereditary Optic Neuropathy. Ann. Neurol. 52, 534–542 (2002).
Kolesnikova, O. A. et al. Suppression of mutations in mitochondrial DNA by tRNAs imported from the cytoplasm. Science 289, 1931–1933 (2000).
Kolesnikova, O. A. et al. Nuclear DNA-encoded tRNAs targeted into mitochondria can rescue a mitochondrial DNA mutation associated with the MERRF syndrome in cultured human cells. Hum. Mol. Genet. 13, 2519–2534 (2004).
Taylor, R. W., Chinnery, P. F., Turnbull, D. M. & Lightowlers, R. N. Selective inhibition of mutant human mitochondrial DNA replication in vitro by peptide nucleic acids. Nature Genet. 15, 212–215 (1997).
Tanaka, M. et al. Gene therapy for mitochondrial disease by delivering restriction endonuclease SmaI into mitochondria. J. Biomed. Sci. 9, 534–541 (2002).
Srivastava, S. & Moraes, C. T. Manipulating mitochondrial DNA heteroplasmy by a mitochondrially targeted restriction endonuclease. Hum. Mol. Genet. 10, 3093–3099 (2001).
Manfredi, G. et al. Oligomycin induces a decrease in the cellular content of a pathogenic mutation in the human mitochondrial ATPase 6 gene. J. Biol. Chem. 274, 9386–9381 (1999).
Santra, S., Gilkerson, R. W., Davidson, M. & Schon, E. A. Ketogenic treatment reduces deleted mitochondrial DNAs in cultured human cells. Ann. Neurol. 56, 662–669 (2004).
Feuermann, M. et al. The yeast counterparts of human 'MELAS' mutations cause mitochondrial dysfunction that can be rescued by overexpression of the mitochondrial translation factor EF-Tu. EMBO Rep. 4, 53–58 (2003).
Harding, A. E., Holt, I. J., Sweeney, M. G., Brockington, M. & Davis, M. B. Prenatal diagnosis of mitochondrial DNA 8993T>G disease. Am. J. Hum. Genet. 50, 629–633 (1992).
Leshinsky-Silver, E. et al. Prenatal exclusion of Leigh syndrome due to T8993C mutation in the mitochondrial DNA. Prenat. Diagn. 23, 31–33 (2003).
Jacobs, L. J. et al. Transmission and prenatal diagnosis of the T9176C mitochondrial DNA mutation. Mol. Hum. Reprod. 11, 223–228 (2005).
Weber, K. et al. A new mtDNA mutation showing accumulation with time and restriction to skeletal muscle. Am. J. Hum. Genet. 60, 373–380 (1997).
White, S. L. et al. Two cases of prenatal analysis for the pathogenic T to G substitution at nucleotide 8993 in mitochondrial DNA. Prenat. Diagn. 19, 1165–1168 (1999).
Lin, D. P. et al. Comparison of mitochondrial DNA contents in human embryos with good or poor morphology at the 8-cell stage. Fertil. Steril. 81, 73–79 (2004).
Dean, N. L. et al. Prospect of preimplantation genetic diagnosis for heritable mitochondrial DNA diseases. Mol. Hum. Reprod. 9, 631–638 (2003).
Kagawa, Y. & Hayashi, J. I. Gene therapy of mitochondrial diseases using human cytoplasts. Gene Ther. 4, 6–10 (1997).
Cohen, J., Scott, R., Schimmel, T., Levron, J. & Willadsen, S. Birth of infant after transfer of anucleate donor oocyte cytoplasm into recipient eggs. Lancet 350, 186–187 (1997).
Hawes, S. M., Sapienza, C. & Latham, K. E. Ooplasmic donation in humans: the potential for epigenic modifications. Hum. Reprod. 17, 850–852 (2002).
Brenner, C. A., Barritt, J. A., Willadsen, S. & Cohen, J. Mitochondrial DNA heteroplasmy after human ooplasmic transplantation. Fertil. Steril. 74, 573–578 (2000).
Thorburn, D. R. & Dahl, H. H. M. Mitochondrial disorders: genetics, counseling, prenatal diagnosis and reproductive options. Am. J. Med. Genet. 106, 102–114 (2001).
Roberts, R. M. Prevention of human mitochondrial (mtDNA) disease by nucleus transplantation into an enucleated donor oocyte. Am. J. Med. Genet. 87, 265–266 (1999). This paper describes the possibility of preventing transmission of mitochondrial DNA disease.
Liu, H., Wang, C. W., Grifo, J. A., Krey, L. C. & Zhang, J. Reconstruction of mouse oocytes by germinal vesicle transfer: maturity of host oocyte cytoplasm determines meiosis. Hum. Reprod. 14, 2357–2361 (1999).
Liu, H., Zhang, J., Krey, L. C. & Grifo, J. A. In-vitro development of mouse zygotes following reconstruction by sequential transfer of germinal vesicles and haploid pronuclei. Hum. Reprod. 15, 1997–2002 (2000).
Takeuchi, T., Ergun, B., Huang, T. H., Rosenwaks, Z. & Palermo, G. D. A reliable technique of nuclear transplantation for immature mammalian oocytes. Hum. Reprod. 14, 1312–1317 (1999).
Barnes, F. L. et al. Blastocyst development and birth after in-vitro maturation of human primary oocytes, intracytoplasmic sperm injection and assisted hatching. Hum. Reprod. 10, 3243–3247 (1995).
Goud, P. T. et al. In-vitro maturation of human germinal vesicle stage oocytes: role of cumulus cells and epidermal growth factor in the culture medium. Hum. Reprod. 13, 1638–1644 (1998).
Kattera, S. & Chen, C. Normal birth after microsurgical enucleation of tripronuclear human zygotes: case report. Hum. Reprod. 18, 1319–1322 (2003).
Meirelles, F. & Smith, L. C. Mitochondrial genotype segregation in a mouse heteroplasmic lineage produced by embyonic karyoplast transplantation. Genetics 145, 445–451 (1997).
Meirelles, F. & Smith, L. C. Mitochondrial genotype segregation during preimplantation development in mouse heteroplasmic embryos. Genetics 148, 877–883 (1998).
Harman, D. Free radical theory of aging. Mutat. Res. 275, 257–266 (1992). This is a description of the theory of ageing in which mitochondria have a key role.
Geromel, V. et al. Superoxide-induced massive apoptosis in cultured skin fibroblasts harboring the neurogenic ataxia retinitis pigmentosa (NARP) mutation in the ATPase-6 gene of the mitochondrial DNA. Hum. Mol. Genet. 10, 1221–1228 (2001).
Mattiazzi, M. et al. The mtDNA T8993G (NARP) mutation results in an impairment of oxidative phosphorylation that can be improved by antioxidants. Hum. Mol. Genet. 13, 869–879 (2004).
Muller-Hocker, J. Cytochrome-c-oxidase deficient cardiomyocytes in the human heart —an age-related phenomenon. A histochemical ultracytochemical study. Am. J. Pathol. 134, 1167–1173 (1989).
Muller-Hocker, J., Seibel, P., Schneiderbanger, K. & Kadenbach, B. Different in situ hybridization patterns of mitochondrial DNA in cytochrome c oxidase-deficient extraocular muscle fibres in the elderly. Virchows Arch. A Pathol. Anat. Histopathol. 422, 7–15 (1993).
Brierley, E. J., Johnson, M. A., Lightowlers, R. N., James, O. F. & Turnbull, D. M. Role of mitochondrial DNA mutations in human aging: implications for the central nervous system and muscle. Ann. Neurol. 43, 217–223 (1998).
Nekhaeva, E. et al. Clonally expanded mtDNA point mutations are abundant in individual cells of human tissues. Proc. Natl Acad. Sci. USA 99, 5521–5526 (2002).
Wallace, D. C. Diseases of the mitochondrial DNA. Annu. Rev. Biochem. 61, 1175–1212 (1992).
de Grey, A. D. A proposed refinement of the mitochondrial free radical theory of aging. Bioessays 19, 161–166 (1997).
Yoneda, M., Chomyn, A., Martinuzzi, A., Hurko, O. & Attardi, G. Marked replicative advantage of human mtDNA carrying a point mutation that causes the MELAS encephalomyopathy. Proc. Natl Acad. Sci. USA 89, 11164–11168 (1992).
Elson, J. L., Samuels, D. C., Turnbull, D. M. & Chinnery, P. F. Random intracellular drift explains the clonal expansion of mitochondrial DNA mutations with age. Am. J. Hum. Genet. 68, 802–806 (2001).
Polyak, K. et al. Somatic mutations of the mitochondrial genome in human colorectal tumours. Nature Genet. 20, 291–293 (1998). The first paper to describe the presence of somatic mtDNA mutations in solid human tumours, in this case colon cancer. In many cases, the mtDNA mutations had accumulated to homoplasmic levels and were not evident in the matched (normal) tissue from the same patient. A causal relationship between mtDNA mutations and tumorigenesis is yet to be established.
Fliss, M. S. et al. Facile detection of mitochondrial DNA mutations in tumors and bodily fluids. Science 287, 2017–2019 (2000).
Jeronimo, C. et al. Mitochondrial mutations in early stage prostate cancer and bodily fluids. Oncogene 20, 5195–5198 (2001).
Jones, J. B. et al. Detection of mitochondrial DNA mutations in pancreatic cancer offers a 'mass'-ive advantage over detection of nuclear DNA mutations. Cancer Res. 61, 1299–1304 (2001).
Kirches, E. et al. High frequency of mitochondrial DNA mutations in glioblastoma multiforme identified by direct sequence comparison to blood samples. Int. J. Cancer 93, 534–538 (2001).
He, L. et al. Somatic mitochondrial DNA mutations in adult-onset leukaemia. Leukemia 17, 2487–2491 (2003).
Wardell, T. M. et al. Changes in the human mitochondrial genome after treatment of malignant disease. Mutat. Res. 525, 19–27 (2003).
Zeviani, M. et al. Deletions of mitochondrial DNA in Kearns–Sayre syndrome. Neurology 38, 1339–1346 (1988).
Goto, Y., Nonaka, I. & Horai, S. A mutation in the tRNALeu(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature 348, 651–653 (1990).
Santorelli, F. M. et al. Identification of a novel mutation in the mtDNA ND5 gene associated with MELAS. Biochem. Biophys. Res. Commun. 238, 326–328 (1997).
Kirby, D. M. et al. Mutations of the mitochondrial ND1 gene as a cause of MELAS. J. Med. Genet. 41, 784–789 (2004).
Shoffner, J. M. et al. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNALys mutation. Cell 61, 931–937 (1990).
Holt, I. J., Harding, A. E., Petty, R. K. & Morgan-Hughes, J. A. A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Am. J. Hum. Genet. 46, 428–433 (1990).
van den Ouweland, J. W. M., Lemkes, H. H. P. J. & Ruitenbeek, K. Mutation in mitochondrial tRNALeu(UUR) gene in a large pedigree with maternally transmitted type II diabetes mellitus and deafness. Nature Genet. 1, 368–371 (1992).
Howell, N. et al. Leber hereditary optic neuropathy: identification of the same mitochondrial ND1 mutation in six pedigrees. Am. J. Hum. Genet. 49, 939–950 (1991).
Johns, D. R., Neufeld, M. J. & Park, R. D. An ND-6 mitochondrial DNA mutation associated with Leber hereditary optic neuropathy. Biochem. Biophys. Res. Commun. 187, 1551–1557 (1992).
Hao, H., Bonilla, E., Manfredi, G., DiMauro, S. & Moraes, C. T. Segregation patterns of a novel mutation in the mitochondrial tRNA glutamic acid gene associated with myopathy and diabetes mellitus. Am. J. Hum. Genet. 56, 1017–1025 (1995).
McFarland, R. et al. Familial myopathy: New insights into the T14709C mitochondrial tRNA mutation. Ann. Neurol. 55, 478–484 (2004).
Reid, F. M., Vernham, G. A. & Jacobs, H. T. A novel mitochondrial point mutation in a maternal pedigree with sensorineural deafness. Hum. Mutat. 3, 243–247 (1994).
Sue, C. M. et al. Maternally inherited hearing loss in a large kindred with a novel T7511C mutation in the mitochondrial DNA tRNASer(UCN) gene. Neurology 52, 1905–1908 (1999).
Strachan, T. & Read, A. P. Human Molecular Genetics 2nd edn (John Wiley and Sons, New York, 1999).
Acknowledgements
The authors work is supported by the Wellcome Trust, Medical Research Centre, Alzheimer's Research Trust, Muscular Dystrophy Campaign, Newcastle upon Tyne Hospitals NHS Trust and the European Union (FP6, EUmitocombat). We thank R.N. Lightowlers, N. Howell, R. McFarland and M. Herbert for valuable input, and L. Craven and M. Barron for help with the figures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Entrez
OMIM
Leber hereditary optic neuropathy
progressive external ophthalmoplegia
FURTHER INFORMATION
Glossary
- NUCLEOID
-
A dynamic complex that consists of several copies of mitochondrial DNA and key maintenance proteins within the organelle.
- POLYCISTRONIC
-
A form of gene organization that results in transcription of an mRNA that codes for multiple gene products, each of which is independently translated from the mRNA.
- TRANSMITOCHONDRIAL CYBRID
-
Derived from the term 'cytoplasmic hybrid'. A cell line that is used to study the pathophysiological consequences of any given mitochondrial DNA mutation in a control nuclear background. Cybrids are generated by fusing patient cytoplasts (enucleated cells that contain mitochondria) with a cell line that lacks mitochondrial DNA.
- LEBER HEREDITARY OPTIC NEUROPATHY
-
A mitochondrial disease that is characterized by optic nerve dysfunction, which leads to bilateral visual failure in young adults.
- GENETIC BOTTLENECK
-
A temporary reduction in population size that causes the loss of genetic variation.
- DIPLOPIA
-
Double vision; derived from the Greek diplous, meaning double, and ops, meaning eye.
- ATAXIA
-
The loss of the ability to coordinate muscular movement.
- DYSPHAGIA
-
A difficulty in swallowing.
- PTOSIS
-
The abnormal lowering or drooping of the upper eyelid that is caused by muscle weakness.
- DYSTONIA
-
A neurological movement disorder that is characterized by involuntary muscle contractions that might cause twisting or jerking movements of the body.
- PANCYTOPAENIA
-
A deficiency of all blood cells including red cells, white cells and platelets.
- OPHTHALMOPLEGIA
-
Weakness of one or more of the muscles that control eye movement.
- PEARSON SYNDROME
-
A severe disease during infancy that affects bone marrow and pancreas function owing to large-scale rearrangements of the mitochondrial genome.
- LEIGH SYNDROME
-
A disease that affects the brainstem and basal ganglia and is characterised by defects in mitochondrial oxidative phosphorylation.
- RHABDOMYOLYSIS
-
The breakdown of muscle fibres owing to injury, toxins or metabolic disease, which leads to high concentrations of myoglobin in both plasma and urine
- RAGGED-RED FIBRES
-
The pathological hallmark of mtDNA disease that is characterized by the subsarcolemmal accumulation of abnormal mitochondria in the muscle fibre, which stains red with a Gomori trichrome stain.
- CYTOPLAST
-
An enucleated donor cell that contains patient mitochondria and that is used to generate cybrid fusions.
- SATELLITE CELLS
-
Quiescent cells that are located between the basal lamina and the plasmalemma of the muscle fibre, and are a main contributor to postnatal muscle growth.
- ALLOTOPIC EXPRESSION
-
The expression of a gene in a different cellular compartment to its target location. In the context described here, it is the recoding of a mitochondrial gene to allow it to be expressed in the nucleus. The subsequent conjugation of a mitochondrial-targeting sequence promotes import and localization of the gene product to the organelle.
- AMNIOCENTESIS
-
An aspiration of cells from the amniotic sac, using a needle, for biochemical and genetic analysis.
- CHORIONIC VILLUS BIOPSY
-
A placental biopsy that is carried out in early pregnancy to collect fetal tissue for genetic and biochemical analysis.
- POLAR BODY
-
A small haploid cell that is produced during oogenesis and that does not develop into a functional ovum.
- GERMINAL VESICLE
-
A stage during oocyte maturation in which the oocyte nucleus is located close to the surface of the egg cell and is clearly visible.
- GENETIC DRIFT
-
Changes in the frequency of a genetic variant in a population owing to chance alone.
Rights and permissions
About this article
Cite this article
Taylor, R., Turnbull, D. Mitochondrial DNA mutations in human disease. Nat Rev Genet 6, 389–402 (2005). https://doi.org/10.1038/nrg1606
Issue Date:
DOI: https://doi.org/10.1038/nrg1606
This article is cited by
-
Strand-selective base editing of human mitochondrial DNA using mitoBEs
Nature Biotechnology (2024)
-
Two mitochondrial DNA polymorphisms modulate cardiolipin binding and lead to synthetic lethality
Nature Communications (2024)
-
Arsenic-Induced Cardiovascular Diseases and their Correlation with Mitochondrial DNA Copy Number, Deletion, and Telomere Length in Bangladeshi Population
Cardiovascular Toxicology (2024)
-
Emerging roles of mitochondria in animal regeneration
Cell Regeneration (2023)
-
A computational study on mitogenome-encoded proteins of Pavo cristatus and Pavo muticus identifies key genetic variations with functional implications
Journal of Genetic Engineering and Biotechnology (2023)