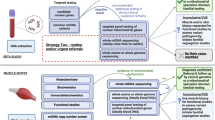
Abstract
Next-generation sequencing (NGS) has increased our understanding of the molecular basis of many primary mitochondrial diseases (PMDs). Despite this progress, many patients with suspected PMD remain without a genetic diagnosis, which restricts their access to in-depth genetic counselling, reproductive options and clinical trials, in addition to hampering efforts to understand the underlying disease mechanisms. Although they represent a considerable improvement over their predecessors, current methods for sequencing the mitochondrial and nuclear genomes have important limitations, and molecular diagnostic techniques are often manual and time consuming. However, recent advances in genomics and transcriptomics offer realistic solutions to these challenges. In this Review, we discuss the current genetic testing approach for PMDs and the opportunities that exist for increased use of whole-genome NGS of nuclear and mitochondrial DNA (mtDNA) in the clinical environment. We consider the possible role for long-read approaches in sequencing of mtDNA and in the identification of novel nuclear genomic causes of PMDs. We examine the expanding applications of RNA sequencing, including the detection of cryptic variants that affect splicing and gene expression and the interpretation of rare and novel mitochondrial transfer RNA variants.
Key points
-
At present, the diagnosis of primary mitochondrial disease is a multistep process often involving a number of time-consuming and highly manual molecular techniques.
-
Early whole-genome sequencing of blood, analysing both mitochondrial and nuclear DNA, is likely to improve diagnostic efficiency in some people with mitochondrial disease.
-
In future, the application of long-read sequencing to mitochondrial DNA could build on the advances made by next-generation sequencing to further enhance coverage, and enable the identification of large-scale rearrangements and point mutations in a single test.
-
As with other rare diseases, whole-genome long-read sequencing might provide the next diagnostic uplift given its ability to identify structural variants, short tandem repeat variants and epigenetic modifications, and to phase compound heterozygous variants.
-
Mitochondrial medicine is poised to benefit substantially from the increasing use of RNA sequencing of tissue samples; advances in pre-processing and sequencing of transfer RNA are enabling new insights into this molecule, which plays an outsized role in the aetiology of mitochondrial diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Rahman, J. & Rahman, S. Mitochondrial medicine in the omics era. Lancet 391, 2560–2574 (2018).
Stenton, S. L. & Prokisch, H. Advancing genomic approaches to the molecular diagnosis of mitochondrial disease. Essays Biochem. 62, 399–408 (2018).
Gorman, G. S. et al. Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Ann. Neurol. 77, 753–759 (2015).
Castro-Gago, M. et al. Epidemiology of pediatric mitochondrial respiratory chain disorders in northwest Spain. Pediatr. Neurol. 34, 204–211 (2006).
Gorman, G. S. et al. Mitochondrial diseases. Nat. Rev. Dis. Primers 2, 16080 (2016).
Carelli, V. & Morgia, C. La. Clinical syndromes associated with mtDNA mutations: where we stand after 30 years. Essays Biochem. 62, 235–254 (2018).
Nesbitt, V. et al. The UK MRC mitochondrial disease patient cohort study: clinical phenotypes associated with the m.3243A>G mutation–implications for diagnosis and management. J. Neurol. Neurosurg. Psychiatry 84, 936–938 (2013).
Pitceathly, R. D. S. et al. Genetic dysfunction of MT-ATP6 causes axonal Charcot-Marie-Tooth disease. Neurology 79, 1145–1154 (2012).
Pitceathly, R., Keshavan, N., Rahman, J. & Rahman, S. Moving towards clinical trials for mitochondrial diseases. J. Inherit. Metab. Dis. https://doi.org/10.1002/jimd.12281 (2020).
Anderson, S. et al. Sequence and organization of the human mitochondrial genome. Nature 290, 1–18 (1981).
Robin, E. D. & Wong, R. Mitochondrial DNA molecules and virtual number of mitochondria per cell in mammalian cells. J. Cell. Physiol. 136, 507–513 (1988).
Bogenhagen, D. F. Mitochondrial DNA nucleoid structure. Biochim. Biophys. Acta 1819, 914–920 (2012).
D’Souza, A. R. & Minczuk, M. Mitochondrial transcription and translation: overview. Essays Biochem. 62, 309–320 (2018).
Wei, W. et al. Nuclear-mitochondrial DNA segments resemble paternally inherited mitochondrial DNA in humans. Nat. Commun. 11, 1–11 (2020).
Rath, S. et al. MitoCarta3.0: an updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Res. 49, D1541–D1547 (2021).
Frazier, A. E., Thorburn, D. R. & Compton, A. G. Mitochondrial energy generation disorders: genes, mechanisms, and clues to pathology. J. Biol. Chem. 294, 5386–5395 (2019).
El-Hattab, A. W., Craigen, W. J. & Scaglia, F. Mitochondrial DNA maintenance defects. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 1539–1555 (2017).
Rahman, S., Poulton, J., Marchington, D. & Suomalainen, A. Decrease of 3243 A→G mtDNA mutation from blood in MELAS syndrome: a longitudinal study. Am. J. Hum. Genet. 68, 238–240 (2001).
Goldstein, A. & Falk, M. J. Mitochondrial DNA deletion syndromes. in GeneReviews (eds Adam, M. P. et al.) (University of Washington, 2019).
He, Y. et al. Heteroplasmic mitochondrial DNA mutations in normal and tumour cells. Nature 464, 610–614 (2010).
Rossignol, R. et al. Mitochondrial threshold effects. Biochem. J. 370, 751–762 (2003).
Maeda, K. et al. Clinical phenotype and segregation of mitochondrial 3243A>G mutation in 2 pairs of monozygotic twins. JAMA Neurol. 73, 990–993 (2016).
Lynn, S., Borthwick, G. M., Charnley, R. M., Walker, M. & Turnbull, D. M. Heteroplasmic ratio of the A3243G mitochondrial DNA mutation in single pancreatic beta cells. Diabetologia 46, 296–299 (2003).
Elliott, H. R., Samuels, D. C., Eden, J. A., Relton, C. L. & Chinnery, P. F. Pathogenic mitochondrial DNA mutations are common in the general population. Am. J. Hum. Genet. 83, 254–260 (2008).
Kohda, M. et al. A comprehensive genomic analysis reveals the genetic landscape of mitochondrial respiratory chain complex deficiencies. PLoS Genet. 12, 1–31 (2016).
Lieber, D. S. et al. Targeted exome sequencing of suspected mitochondrial disorders. Neurology 80, 1762–1770 (2013).
Ohtake, A. et al. Diagnosis and molecular basis of mitochondrial respiratory chain disorders: exome sequencing for disease gene identification. Biochim. Biophys. Acta Gen. Subj. 1840, 1355–1359 (2014).
Pronicka, E. et al. New perspective in diagnostics of mitochondrial disorders: two years’ experience with whole-exome sequencing at a national paediatric centre. J. Transl. Med. 14, 1–19 (2016).
Taylor, R. W. et al. Use of whole-exome sequencing to determine the genetic basis of multiple mitochondrial respiratory chain complex deficiencies. JAMA 312, 68–77 (2014).
Wortmann, S. B., Koolen, D. A., Smeitink, J. A., van den Heuvel, L. & Rodenburg, R. J. Whole exome sequencing of suspected mitochondrial patients in clinical practice. J. Inherit. Metab. Dis. 38, 437–443 (2015).
Cui, H. et al. Comprehensive next-generation sequence analyses of the entire mitochondrial genome reveal new insights into the molecular diagnosis of mitochondrial DNA disorders. Genet. Med. 15, 388–394 (2013).
Zhang, W., Cui, H. & Wong, L. J. C. Comprehensive one-step molecular analyses of mitochondrial genome by massively parallel sequencing. Clin. Chem. 58, 1322–1331 (2012). The key paper that established the role of deep NGS of long-range PCR-amplified mtDNA in identifying point mutations and large deletions, including heteroplasmic variants.
Seneca, S. et al. Analysis of the whole mitochondrial genome: translation of the Ion Torrent Personal Genome Machine system to the diagnostic bench? Eur. J. Hum. Genet. 23, 41–48 (2015).
Riley, L. G. et al. The diagnostic utility of genome sequencing in a pediatric cohort with suspected mitochondrial disease. Genet. Med. 22, 1254–1261 (2020). The first study to use WGS specifically for mitochondrial presentations.
Kremer, L. S. et al. Genetic diagnosis of Mendelian disorders via RNA sequencing. Nat. Commun. 8, 1–11 (2017). This paper shows that RNA-seq can be used to diagnose mitochondrial diseases and is the first large study to demonstrate the clinical utility of the technique in a rare disease.
Ellerby, L. M. Repeat expansion disorders: mechanisms and therapeutics. Neurotherapeutics 16, 924–927 (2019).
Yu-Wai-Man, P. & Chinnery, P. F. Leber hereditary optic neuropathy. in GeneReviews (eds Adam, M. P. et al.) 1–19 (University of Washington, Seattle, 2016).
Wong, L. J. C. et al. Utility of oligonucleotide array-based comparative genomic hybridization for detection of target gene deletions. Clin. Chem. 54, 1141–1148 (2008).
Naini, A. & Shanske, S. Detection of mutations in mtDNA. Methods Cell Biol. 80, 437–463 (2007).
Chinault, A. C., Shaw, C. A., Brundage, E. K., Tang, L. Y. & Wong, L. J. C. Application of dual-genome oligonucleotide array-based comparative genomic hybridization to the molecular diagnosis of mitochondrial DNA deletion and depletion syndromes. Genet. Med. 11, 518–526 (2009).
Shanske, S. & Wong, L. J. C. Molecular analysis for mitochondrial DNA disorders. Mitochondrion 4, 403–415 (2004).
Grier, J., Hirano, M., Karaa, A., Shepard, E. & Thompson, J. L. P. Diagnostic odyssey of patients with mitochondrial disease results of a survey. Neurol. Genet. 4, e230 (2018).
Alston, C. L., Rocha, M. C., Lax, N. Z., Turnbull, D. M. & Taylor, R. W. The genetics and pathology of mitochondrial disease. J. Pathol. 241, 236–250 (2017).
Wong, L. J. C. et al. Interpretation of mitochondrial tRNA variants. Genet. Med. 22, 917–926 (2020). An insightful paper that presents the analysis of a large number of mt-tRNA variants in the context of modern variant classification standards and suggests a tailored approach to classifying mutations in this unique group of molecules.
Diroma, M. A. et al. Extraction and annotation of human mitochondrial genomes from 1000 Genomes Whole Exome Sequencing data. BMC Genomics 15, 1–15 (2014).
Garret, P. et al. Deciphering exome sequencing data: bringing mitochondrial DNA variants to light. Hum. Mutat. 40, 2430–2443 (2019).
Griffin, H. R. et al. Accurate mitochondrial DNA sequencing using off-target reads provides a single test to identify pathogenic point mutations. Genet. Med. 16, 962–971 (2014).
van Oven, M. & Kayser, M. Updated comprehensive phylogenetic tree of global human mitochondrial DNA variation. Hum. Mutat. 30, 386–394 (2009).
Ghelli, A. M. et al. The background of mitochondrial DNA haplogroup J increases the sensitivity of Leber’s hereditary optic neuropathy cells to 2,5-hexanedione toxicity. PLoS ONE 4, e7922 (2009).
Hudson, G. et al. Clinical expression of leber hereditary optic neuropathy is affected by the mitochondrial DNA-haplogroup background. Am. J. Hum. Genet. 81, 228–233 (2007).
Ye, F., Samuels, D. C., Clark, T. & Guo, Y. High-throughput sequencing in mitochondrial DNA research. Mitochondrion 17, 157–163 (2014).
Gould, M. P. et al. PCR-free enrichment of mitochondrial DNA from human blood and cell lines for high quality next-generation DNA sequencing. PLoS ONE 10, 1–13 (2015).
Akbari, M., Hansen, M. D., Halgunset, J., Skorpen, F. & Krokan, H. E. Low copy number DNA template can render polymerase chain reaction error prone in a sequence-dependent manner. J. Mol. Diagn. 7, 36–39 (2005).
Santibanez-koref, M. et al. Assessing mitochondrial heteroplasmy using next generation sequencing: a note of caution. Mitochondrion 46, 302–306 (2019).
Hazkani-Covo, E., Zeller, R. M. & Martin, W. Molecular poltergeists: mitochondrial DNA copies (numts) in sequenced nuclear genomes. PLoS Genet. 6, e1000834 (2010).
Tourmen, Y. et al. Structure and chromosomal distribution of human mitochondrial pseudogenes. Genomics 80, 71–77 (2002).
Parr, R. L. et al. The pseudo-mitochondrial genome influences mistakes in heteroplasmy interpretation. BMC Genomics 13, 1–13 (2006).
Marquis, J. et al. MitoRS, a method for high throughput, sensitive, and accurate detection of mitochondrial DNA heteroplasmy. BMC Genomics 18, 1–19 (2017).
Wolff, J. N., Shearman, D. C. A., Brooks, R. C. & Ballard, J. W. O. Selective enrichment and sequencing of whole mitochondrial genomes in the presence of nuclear encoded mitochondrial pseudogenes (Numts). PLoS ONE 7, 1–7 (2012).
Ancora, M. et al. Mitochondrial heteroplasmy profiling in single human oocytes by next-generation sequencing. Mitochondrial DNA B Resour 2, 543–544 (2017).
Yao, Y. et al. A simple method for sequencing the whole human mitochondrial genome directly from samples and its application to genetic testing. Sci. Rep. 9, 1–7 (2019).
Williams, S. L. et al. The mtDNA mutation spectrum of the progeroid Polg mutator mouse includes abundant control region multimers. Cell Metab. 12, 675–682 (2010).
Maricic, T., Whitten, M. & Pa, S. Multiplexed DNA sequence capture of mitochondrial genomes using PCR products. PLoS ONE 5, 9–13 (2010).
Weerts, M. J. A. et al. Sensitive detection of mitochondrial DNA variants for analysis of mitochondrial DNA-enriched extracts from frozen tumor tissue. Sci. Rep. 8, 2261 (2018).
McDowell, D. G., Burns, N. A. & Parkes, H. C. Localised sequence regions possessing high melting temperatures prevent the amplification of a DNA mimic in competitive PCR. Nucleic Acids Res. 26, 3340–3347 (1998).
Wood, E. et al. Clinical long-read sequencing of the human mitochondrial genome for mitochondrial disease diagnostics. Preprint at bioRxiv https://www.biorxiv.org/content/10.1101/597187v1 (2019). The first clinical use of nanopore for mtDNA variants; although accuracy problems lead to false-positive point mutations, the technology successfully sequenced the entire genome and identified deletions missed by NGS.
Strachan, T. & Read, A. Human Molecular Genetics (CRC Press, 2019).
Almannai, M., El-Hattab, A. W. & Scaglia, F. Mitochondrial DNA replication: clinical syndromes. Essays Biochem. 62, 297–308 (2018).
Raymond, F. L., Horvath, R. & Chinnery, P. F. First-line genomic diagnosis of mitochondrial disorders. Nat. Rev. Genet. 19, 399–400 (2018).
Koenig, M. K. Presentation and diagnosis of mitochondrial disorders in children. Pediatr. Neurol. 38, 305–313 (2008).
Posey, J. E. et al. Resolution of disease phenotypes resulting from multilocus genomic variation. N. Engl. J. Med. 376, 21–31 (2017).
Parikh, S. et al. Diagnosis of possible mitochondrial disease: an existential crisis. J. Med. Genet. 56, 123–130 (2019).
Uittenbogaard, M. et al. The nuclear background influences the penetrance of the near-homoplasmic m.1630 A>G MELAS variant in a symptomatic proband and asymptomatic mother. Mol. Genet. Metab. 126, 429–438 (2019).
Boczonadi, V., Bansagi, B. & Horvath, R. Reversible infantile mitochondrial diseases. J. Inherit. Metab. Dis. 38, 427–435 (2015).
Horvath, R. et al. Molecular basis of infantile reversible cytochrome c oxidase deficiency myopathy. Brain 132, 3165–3174 (2009).
Hathazi, D. et al. Metabolic shift underlies recovery in reversible infantile respiratory chain deficiency. EMBO J. 44, 1–19 (2020). A very recent paper that elegantly illustrates the complex interplay between the mitochondrial and nuclear genomes.
Rygiel, K. A. et al. Complex mitochondrial DNA rearrangements in individual cells from patients with sporadic inclusion body myositis. Nucleic Acids Res. 44, 5313–5329 (2016).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–424 (2015).
McCormick, E. M. et al. Specifications of the ACMG/AMP standards and guidelines for mitochondrial DNA variant interpretation Hum. Mutat. 41, 2028–2057 (2020).
Marshall, C. R. et al. The medical genome initiative: moving whole-genome sequencing for rare disease diagnosis to the clinic. Genome Med. 12, 48 (2020).
Gerner-Smidt, P. et al. Whole genome sequencing: bridging one-health surveillance of foodborne diseases. Front. Public Health 7, 1–11 (2019).
Giannopoulou, E., Katsila, T., Mitropoulou, C., Tsermpini, E. E. & Patrinos, G. P. Integrating next-generation sequencing in the clinical pharmacogenomics workflow. Front. Pharmacol. 10, 1–6 (2019).
Turnbull, C. et al. The 100 000 Genomes Project: bringing whole genome sequencing to the NHS. BMJ 361, 1–7 (2018).
Handsaker, R. E. et al. Large multiallelic copy number variations in humans. Nat. Genet. 47, 296–303 (2015).
Sudmant, P. H. et al. An integrated map of structural variation in 2,504 human genomes. Nature 526, 75–81 (2015).
Eid, J. et al. Real-time DNA sequencing from single polymerase molecules. Science 323, 133–138 (2009).
Travers, K. J., Chin, C. S., Rank, D. R., Eid, J. S. & Turner, S. W. A flexible and efficient template format for circular consensus sequencing and SNP detection. Nucleic Acids Res. 38, e159 (2010).
van Dijk, E. L., Jaszczyszyn, Y., Naquin, D. & Thermes, C. The third revolution in sequencing technology. Trends Genet. 34, 666–681 (2018).
Amarasinghe, S. L. et al. Opportunities and challenges in long-read sequencing data analysis. Genome Biol. 21, 1–16 (2020).
Vossen, R. H. A. M. & Buermans, H. P. J. in Genotyping: Methods and Protocols (eds White, S. J. & Cantsilieris, S.) 179–184 (Humana Press, 2017).
Chakraborty, S. et al. Mitochondrial DNA sequencing using PacBio SMRT technology. Presented at the PacBio Advances in Genome Biology and Technology conference. (2018).
Borràs, D. M. et al. Detecting PKD1 variants in polycystic kidney disease patients by single-molecule long-read sequencing. Hum. Mutat. 38, 870–879 (2017).
Frans, G. et al. Conventional and single-molecule targeted sequencing method for specific variant detection in IKBKG while bypassing the IKBKGP1 pseudogene. J. Mol. Diagn. 20, 195–202 (2018).
Alkanaq, A. N. et al. Comparison of mitochondrial DNA variants detection using short- and long-read sequencing. J. Hum. Genet. 64, 1107–1116 (2019).
Wenger, A. M. et al. Accurate circular consensus long-read sequencing improves variant detection and assembly of a human genome. Nat. Biotechnol. 37, 1155–1162 (2019).
Vollger, M. R. et al. Improved assembly and variant detection of a haploid human genome using single-molecule, high-fidelity long reads. Ann. Hum. Genet. 84, 125–140 (2020).
Zascavage, R. R. et al. Approaches to whole mitochondrial genome sequencing on the Oxford Nanopore MinION. Curr. Protoc. Hum. Genet. 104, e94 (2019).
Zascavage, R. R., Thorson, K. & Planz, J. V. Nanopore sequencing: an enrichment-free alternative to mitochondrial DNA sequencing. Electrophoresis 40, 272–280 (2019).
Jain, M. et al. Nanopore sequencing and assembly of a human genome with ultra-long reads. Nat. Biotechnol. 36, 338–345 (2018).
Chaisson, M. J. P. et al. Resolving the complexity of the human genome using single-molecule sequencing. Nature 517, 608–611 (2015).
Seo, J. S. et al. De novo assembly and phasing of a Korean human genome. Nature 538, 243–247 (2016).
Sharp, A. J. et al. Segmental duplications and copy-number variation in the human genome. Am. J. Hum. Genet. 77, 78–88 (2005).
Chiang, C. et al. The impact of structural variation on human gene expression. Nat. Genet. 49, 692–699 (2017).
Chaisson, M. J. P. et al. Multi-platform discovery of haplotype-resolved structural variation in human genomes. Nat. Commun. 10, 1–16 (2019).
Beyter, D. et al. Long read sequencing of 1,817 Icelanders provides insight into the role of structural variants in human disease. Preprint at bioRxiv https://www.biorxiv.org/content/10.1101/848366v2 (2019).
Dolzhenko, E. et al. ExpansionHunter: a sequence-graph-based tool to analyze variation in short tandem repeat regions. Bioinformatics 35, 4754–4756 (2019).
Dolzhenko, E. et al. ExpansionHunter Denovo: a computational method for locating known and novel repeat expansions in short-read sequencing data. Genome Biol. 21, 1–14 (2020).
Mitsuhashi, S. et al. Tandem-genotypes: robust detection of tandem repeat expansions from long DNA reads. Genome Biol. 20, 1–17 (2019).
Ummat, A. & Bashir, A. Resolving complex tandem repeats with long reads. Bioinformatics 30, 3491–3498 (2014).
Liu, Q., Zhang, P., Wang, D., Gu, W. & Wang, K. Interrogating the ‘unsequenceable’ genomic trinucleotide repeat disorders by long-read sequencing. Genome Med. 9, 1–16 (2017).
Sone, J. et al. Long-read sequencing identifies GGC repeat expansions in NOTCH2NLC associated with neuronal intranuclear inclusion disease. Nat. Genet. 51, 1215–1221 (2019).
Ardui, S. et al. Detecting AGG interruptions in females with a FMR1 premutation by long-read single-molecule sequencing: a 1 year clinical experience. Front. Genet. 9, 1–6 (2018).
Cumming, S. A. et al. De novo repeat interruptions are associated with reduced somatic instability and mild or absent clinical features in myotonic dystrophy type 1. Eur. J. Hum. Genet. 26, 1635–1647 (2018).
Nakamura, H. et al. Long-read sequencing identifies the pathogenic nucleotide repeat expansion in RFC1 in a Japanese case of CANVAS. J. Hum. Genet. 65, 475–480 (2020).
Mitsuhashi, S. et al. Nanopore-based single molecule sequencing of the D4Z4 array responsible for facioscapulohumeral muscular dystrophy. Sci. Rep. 7, 1–8 (2017).
Nageshwaran, S. & Festenstein, R. Epigenetics and triplet-repeat neurological diseases. Front. Neurol. 6, 1–9 (2015).
Elhamamsy, A. R. Role of DNA methylation in imprinting disorders: an updated review. J. Assist. Reprod. Genet. 34, 549–562 (2017).
Sadikovic, B., Aref-Eshghi, E., Levy, M. A. & Rodenhiser, D. DNA methylation signatures in mendelian developmental disorders as a diagnostic bridge between genotype and phenotype. Epigenomics 11, 563–575 (2019).
Sharma, N., Pasala, M. S. & Prakash, A. Mitochondrial DNA: epigenetics and environment. Environ. Mol. Mutagen. 60, 668–682 (2019).
Patil, V. et al. Human mitochondrial DNA is extensively methylated in a non-CpG context. Nucleic Acids Res. 47, 10072–10085 (2019).
Pearce, S. F. et al. Regulation of mammalian mitochondrial gene expression: recent advances. Trends Biochem. Sci. 42, 625–639 (2017).
Kang, D., Miyako, K., Kai, Y., Irie, T. & Takeshige, K. In vivo determination of replication origins of human mitochondrial DNA by ligation-mediated polymerase chain reaction. J. Biol. Chem. 272, 15275–15279 (1997).
Emrich, S. J., Barbazuk, W. B., Li, L. & Schnable, P. S. Gene discovery and annotation using LCM-454 transcriptome sequencing. Genome Res. 17, 69–73 (2007).
Lister, R. et al. Highly integrated single-base resolution maps of the epigenome in Arabidopsis. Cell 133, 523–536 (2008).
Stark, R., Grzelak, M. & Hadfield, J. RNA sequencing: the teenage years. Nat. Rev. Genet. 20, 631–656 (2019).
Garalde, D. R. et al. Highly parallel direct RNA sequencing on an array of nanopores. Nat. Methods 15, 201–206 (2018).
Workman, R. E. et al. Nanopore native RNA sequencing of a human poly(A) transcriptome. Nat. Methods 16, 1297–1308 (2019).
Navarro-Sastre, A. et al. A fatal mitochondrial disease is associated with defective NFU1 function in the maturation of a subset of mitochondrial Fe-S proteins. Am. J. Hum. Genet. 89, 656–667 (2011).
Pitceathly, R. D. S. et al. NDUFA4 mutations underlie dysfunction of a cytochrome c oxidase subunit linked to human neurological disease. Cell Rep. 3, 1795–1805 (2013).
Taanman, J. W. et al. Characterization of a novel TYMP splice site mutation associated with mitochondrial neurogastrointestinal encephalomyopathy (MNGIE). Neuromuscul. Disord. 19, 151–154 (2009).
López-Bigas, N., Audit, B., Ouzounis, C., Parra, G. & Guigó, R. Are splicing mutations the most frequent cause of hereditary disease? FEBS Lett. 579, 1900–1903 (2005).
Lord, J. et al. Pathogenicity and selective constraint on variation near splice sites. Genome Res. 29, 159–170 (2019).
Wai, H. A. et al. Blood RNA analysis can increase clinical diagnostic rate and resolve variants of uncertain significance. Genet. Med. 22, 1005–1014 (2020).
Cummings, B. B. et al. Improving genetic diagnosis in Mendelian disease with transcriptome sequencing. Sci. Transl. Med. 12, 1–25 (2017).
Gonorazky, H. D. et al. Expanding the boundaries of RNA sequencing as a diagnostic tool for rare mendelian disease. Am. J. Hum. Genet. 104, 466–483 (2019).
Frésard, L. et al. Identification of rare-disease genes using blood transcriptome sequencing and large control cohorts. Nat. Med. 25, 911–919 (2019).
Tyynismaa, H. et al. Mitochondrial myopathy induces a starvation-like response. Hum. Mol. Genet. 19, 3948–3958 (2010).
Deng, J. et al. RNA-seq profiling, and impaired autophagic process in skeletal muscle of MELAS. Biochem. Biophys. Res. Commun. 523, 91–97 (2020).
Gao, S. et al. Mitochondrion two novel lncRNAs discovered in human mitochondrial DNA using PacBio full-length transcriptome data. Mitochondrion 38, 41–47 (2018).
Shoffner, J. M. et al. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNALys mutation. Cell 61, 931–937 (1990).
Goto, Y. I., Nonaka, I. & Horai, S. A mutation in the tRNALeu(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature 348, 651–653 (1990).
DiMauro, S. & Garone, C. Historical perspective on mitochondrial medicine. Dev. Disabil. Res. Rev. 16, 106–113 (2010).
Pan, T. Modifications and functional genomics of human transfer RNA. Cell Res. 28, 395–404 (2018).
Boczonadi, V., Ricci, G. & Horvath, R. Mitochondrial DNA transcription and translation: clinical syndromes. Essays Biochem. 62, 321–340 (2018).
Yarham, J. W., Elson, J. L., Blakely, E. L., Mcfarland, R. & Taylor, R. W. Mitochondrial tRNA mutations and disease. Wiley Interdiscip. Rev. RNA 1, 304–324 (2010).
Wong, L.-J. C. et al. Comprehensive scanning of the entire mitochondrial genome for mutations. Clin. Chem. 48, 1901–1912 (2002).
Zheng, G. et al. Efficient and quantitative high-throughput tRNA sequencing. Nat. Methods 12, 835–837 (2015).
Wilusz, J. E. Removing roadblocks to deep sequencing of modified RNAs. Nat. Methods 12, 821–822 (2015).
Gogakos, T. et al. Characterizing expression and processing of precursor and mature human tRNAs by hydro-tRNAseq and PAR-CLIP. Cell Rep. 20, 1463–1475 (2017).
Cozen, A. E. et al. ARM-seq: AlkB-facilitated RNA methylation sequencing reveals a complex landscape of modified tRNA fragments. Nat. Methods 12, 879–884 (2015).
Shigematsu, M. et al. YAMAT-seq: an efficient method for high-throughput sequencing of mature transfer RNAs. Nucleic Acids Res. 45, e70 (2017).
Pinkard, O., McFarland, S., Sweet, T. & Coller, J. Quantitative tRNA-sequencing uncovers metazoan tissue-specific tRNA regulation. Nat. Commun. 11, 1–15 (2020).
James-Bott, A. & Cribbs, A. P. tRNAnalysis: a flexible pre-processing and next-generation sequencing data analysis pipeline for transfer RNA. Preprint at bioRxiv https://www.biorxiv.org/content/10.1101/655829v1 (2019)
Smith, A. M., Abu-Shumays, R., Akeson, M. & Bernick, D. L. Capture, unfolding, and detection of individual tRNA molecules using a nanopore device. Front. Bioeng. Biotechnol. 3, 1–11 (2015).
Richter, U. et al. RNA modification landscape of the human mitochondrial tRNALys regulates protein synthesis. Nat. Commun. 9, 1–11 (2018).
Metodiev, M. D. et al. Recessive mutations in TRMT10C cause defects in mitochondrial RNA processing and multiple respiratory chain deficiencies. Am. J. Hum. Genet. 98, 993–1000 (2016).
Holzmann, J. et al. RNase P without RNA: identification and functional reconstitution of the human mitochondrial tRNA processing enzyme. Cell 135, 462–474 (2008).
Lehmann, D. et al. Pathogenic mitochondrial mt-tRNA Ala variants are uniquely associated with isolated myopathy. Eur. J. Hum. Genet. 23, 1735–1738 (2015).
Taylor, R. W. et al. A homoplasmic mitochondrial transfer ribonucleic acid mutation as a cause of maternally inherited hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 41, 1786–1796 (2003).
Meseguer, S. et al. The MELAS mutation m.3243 A>G alters the expression of mitochondrial tRNA fragments. Biochim. Biophys. Acta Mol. Cell Res. 1866, 1433–1449 (2019).
El-Hattab, A. W., Almannai, M. & Scaglia, F. MELAS. in GeneReviews (eds Adam, M. P. et al.) (University of Washington, 2001).
Naing, A. et al. Maternally inherited diabetes and deafness (MIDD): diagnosis and management. J. Diabetes Complications 28, 542–546 (2014).
Moraes, C. T. et al. Atypical clinical presentations associated with the MELAS mutation at position 3243 of human mitochondrial DNA. Neuromuscul. Disord. 3, 43–50 (1993).
Horga, A. et al. Peripheral neuropathy predicts nuclear gene defect in patients with mitochondrial ophthalmoplegia. Brain 137, 3200–3212 (2014).
DiMauro, S. & Hirano, M. MERRF. in GeneReviews (eds Adam, M. P. et al.) (University of Washington, 2003).
Rahman, S. et al. Leigh syndrome: clinical features and biochemical and DNA abnormalities. Ann. Neurol. 39, 343–351 (1996).
Thorburn, D. R., Rahman, J. & Rahman, S. Mitochondrial DNA-associated Leigh syndrome and NARP. in GeneReviews (eds Adam, M. P. et al.) (University of Washington, 2003).
Acknowledgements
The University College London Hospitals/University College London Queen Square Institute of Neurology sequencing facility receives a proportion of funding from the Department of Health’s National Institute for Health Research Biomedical Research Centres funding scheme. The clinical and diagnostic ‘Rare Mitochondrial Disorders’ Service in London is funded by the UK NHS Highly Specialized Commissioners. The work of R.D.S.P. is supported by a Medical Research Council Clinician Scientist Fellowship (MR/S002065/1). J.V. holds a fellowship from the Health Education England Genomics Education Programme. All authors are supported by a Medical Research Council strategic award to establish an International Centre for Genomic Medicine in Neuromuscular Diseases (ICGNMD) (MR/S005021/1). The authors are grateful for the feedback on the manuscript provided by L. Wilson, research manager for the ICGNMD, and for the expert input from R. Labrum and C. Woodward, Neurogenetics Unit, The National Hospital for Neurology and Neurosurgery, London.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the conceptualization and writing of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Neurology thanks D. Ghezzi, M. Hirano, E. Morava-Kozicz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Genomics England PanelApp: https://panelapp.genomicsengland.co.uk
MITOMAP: https://www.mitomap.org/MITOMAP
Supplementary information
Glossary
- Polycistronic transcript
-
A transcript that contains the code for more than one polypeptide.
- mtDNA maintenance
-
Continuous re-synthesis of mtDNA by a nuclear-encoded replication apparatus supported by a sustained pool of mitochondrial nucleotides.
- Postmitotic tissue
-
Tissues, such as muscle, that are terminally differentiated and no longer replicate, and therefore are more likely to retain pathogenic mtDNA variants than mitotic tissue.
- Deep sequencing
-
Sequencing a DNA locus many more times than in standard NGS, enabling low levels of alternative alleles (heteroplasmy and mosaicism) to be identified.
- Next-generation sequencing
-
(NGS). A process by which DNA is fragmented into short molecules and denatured; millions of sequencing reactions (addition of fluorescence-labelled nucleotides to form a complementary strand) then occur concurrently and the short sequences or ‘reads’ generated are mapped to a reference genome.
- Short reads
-
The fragments of genetic sequence generated in NGS; typically ~150 bp in length.
- Structural variants
-
(SVs). Large genetic variants such as copy number variants (deletions and duplications), inversions, and translocations, typically >1,000 bp.
- Phase
-
The homologous chromosome of origin (either maternal or paternal).
- Epigenetic modifications
-
Chemical modifications to DNA or the histone molecules around which DNA is packaged; they do not change the genetic code, but can alter gene expression.
- Whole-exome trio
-
Sequencing and comparison of the coding DNA of an affected proband and the proband’s unaffected parents.
- Restriction fragment length polymorphism
-
Differences between individuals in the length of DNA fragments produced by restriction enzymes; the presence of a mutation can create or remove a restriction site.
- mtDNA large-scale rearrangement
-
A rearrangement, typically a deletion and/or duplication, of >1,000 bp in mitochondrial DNA.
- Long-range PCR
-
PCR amplification of mtDNA as one or two fragments using specialized polymerase; traditional PCR amplifies shorter fragments of DNA.
- Coverage
-
Refers to the adequate sequencing of a locus; targeted sequencing can have poor uniformity of coverage.
- GC content
-
Proportion of bases that are guanine–cytosine.
- Phenocopies
-
Diseases with clinical presentations that overlap substantially with the disease of interest.
- De novo assembly
-
Assembly of reads into a continuous sequence without the need to align them against a reference sequence.
- Homopolymeric regions
-
Sequences of DNA comprising identical repeated units of sequence.
- Contigs
-
Consensus sequences comprising overlapping short sequence reads.
- Imprinted genes
-
Genes that are expressed from only one parental origin; the silenced parental copy is said to be ‘imprinted’.
- Adaptor ligation
-
A short synthetic DNA molecule added to the end of the DNA fragment to enable sequencing of that fragment.
- Anticodon
-
The three-nucleotide sequence in tRNA, which is complementary to a codon in mRNA.
- Wobble position
-
The third nucleotide in the anticodon; the Watson–Crick base pairing here is less specific than usual and atypical pairing can occur.
- Cybrids
-
Cell lines created by fusing an enucleated cell (only containing mtDNA) with a nucleated cell, which can contain nDNA and mtDNA or be modified to contain only nDNA.
Rights and permissions
About this article
Cite this article
Macken, W.L., Vandrovcova, J., Hanna, M.G. et al. Applying genomic and transcriptomic advances to mitochondrial medicine. Nat Rev Neurol 17, 215–230 (2021). https://doi.org/10.1038/s41582-021-00455-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-021-00455-2
This article is cited by
-
Nanopore DNA sequencing technologies and their applications towards single-molecule proteomics
Nature Chemistry (2024)
-
Wide diagnostic and genotypic spectrum in patients with suspected mitochondrial disease
Orphanet Journal of Rare Diseases (2023)
-
A method for multiplexed full-length single-molecule sequencing of the human mitochondrial genome
Nature Communications (2022)
-
Specialist multidisciplinary input maximises rare disease diagnoses from whole genome sequencing
Nature Communications (2022)
-
Mitochondrial DNA variants in genomic data: diagnostic uplifts and predictive implications
Nature Reviews Genetics (2021)