Abstract
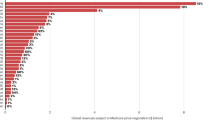
The past 60 years have seen huge advances in many of the scientific, technological and managerial factors that should tend to raise the efficiency of commercial drug research and development (R&D). Yet the number of new drugs approved per billion US dollars spent on R&D has halved roughly every 9 years since 1950, falling around 80-fold in inflation-adjusted terms. There have been many proposed solutions to the problem of declining R&D efficiency. However, their apparent lack of impact so far and the contrast between improving inputs and declining output in terms of the number of new drugs make it sensible to ask whether the underlying problems have been correctly diagnosed. Here, we discuss four factors that we consider to be primary causes, which we call the 'better than the Beatles' problem; the 'cautious regulator' problem; the 'throw money at it' tendency; and the 'basic research–brute force' bias. Our aim is to provoke a more systematic analysis of the causes of the decline in R&D efficiency.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hogan, J. C. Combinatorial chemistry in drug discovery. Nature Biotech. 15, 328–330 (1997).
Geysen, H. M., Schoenen, F., Wagner, D. & Wagner, R. Combinatorial compound libraries for drug discovery: an ongoing challenge. Nature Rev. Drug Discov. 2, 222–230 (2003).
[No authors listed.] Combinatorial chemistry. Nature Biotech. 18, IT50–IT52 (2000).
Dolle, R. E. Historical overview of chemical library design. Methods Mol. Biol. 685, 3–25 (2011).
Sanger, F. Sequences, sequences, and sequences. Annu. Rev. Biochem. 57, 1–28 (1988).
Sanger, F. et al. Nucleotide sequence of bacteriophage phi X174 DNA. Nature 265, 687–695 (1977).
Meldrum, C., Doyle, M. A. & Tothill, R. W. Next-generation sequencing for cancer diagnostics: a practical perspective. Clin. Biochem. Rev. 32, 177–195 (2011).
Joachimiak, A. High-throughput crystallography for structural genomics. Curr. Opin. Struct. Biol. 19, 573–584 (2009).
Van Brunt, J. Protein architecture: designing from the ground up. Nature Biotech. 4, 277–283 (1986).
Mayr, L. M. & Fuerst, P. The future of high-throughput screening. J. Biomol. Screen. 13, 443–448 (2008).
Schnee, J. E. Development cost: determinants and overruns. J. Bus. 45, 347–374 (1972).
Baily, M. N. Research and development costs and returns: the U.S. pharmaceutical industry. J. Polit. Econ. 80, 70–85 (1972).
Comanor, W. Research and technical change in the pharmaceutical industry. Rev. Econ. Stat. 47, 182–190 (1965).
Grabowski, H. G., Vernon, J. M. & Thomas, L. G. Estimating the effects of regulation on innovation: an international comparative analysis of the pharmaceutical industry. J. Law Econ. 21, 133–165 (1978).
Grabowski, H. & Vernon, J. A new look at the returns and risks to pharmaceutical R&D. Manage. Sci. 36, 804–821 (1990).
Jensen, E. J. Research expenditures and the discovery of new drugs. J. Ind. Econ. 36, 83–95 (1987).
Joglekar, P. & Paterson, M. L. A closer look at the returns and risks of pharmaceutical R&D. J. Health Econ. 5, 153–177 (1986).
Elias, T., Gordian, M., Singh, N. & Zemmel, R. Why products fail in Phase III. In Vivo 24, 49–56 (2006).
Pammolli, F., Magazzini, L. & Riccaboni, M. The productivity crisis in pharmaceutical R&D. Nature Rev. Drug Discov. 10, 428–438 (2011).
Kola, I. & Landis, J. Can the pharmaceutical industry reduce attrition rates? Nature Rev. Drug Discov. 3, 711–715 (2004).
DiMasi, J. A., Feldman, L., Seckler, A. & Wilson, A. Trends in risks associated with new drug development: success rates for investigational drugs. Clin. Pharmacol. Ther. 87, 272–277 (2010).
Paul, S. M. et al. How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nature Rev. Drug Discov. 9, 203–214 (2010).
US Food and Drug Administration. Innovation or Stagnation: Challenge and Opportunity on the Critical Path to New Medical Products. FDA website [online], (2004).
Munos, B. Lessons from 60 years of pharmaceutical innovation. Nature Rev. Drug Discov. 8, 959–968 (2010).
Borhani, D. W. & Butts, J. A. Rethinking clinical trials: biology's mysteries. Science 334, 1346–1347 (2011).
David, E., Tramontin, T. & Zemmel, R. Pharmaceutical R&D: the road to positive returns. Nature Rev. Drug Discov. 8, 609–610 (2009).
Garnier, J. P. Rebuilding the R&D engine in big pharma. Harv. Bus. Rev. 86, 68–79 (2008).
Agarwal, S. et al. Unlocking the value in big pharma. McKinsey Quarterly 2, 65–73 (2001).
Ruffolo, R. R. Engineering success: Wyeth redefines its research & development organisation. Drug Discovery World website [online], (2005).
Douglas, F. L., Narayanan, V. K., Mitchell, L. & Litan, R. E. The case for entrepreneurship in R&D in the pharmaceutical industry. Nature Rev. Drug Discov. 9, 683–689 (2010).
Zhong, X. & Moseley, G. B. Mission possible: managing innovation in drug discovery. Nature Biotech. 25, 945–946 (2007).
Horrobin, D. Realism in drug discovery — could Cassandra be right? Nature Biotech. 19, 1099–1100 (2001).
Horrobin, D. F. Innovation in the pharmaceutical industry. J. R. Soc. Med. 93, 341–345 (2000).
Horrobin, D. F. Modern biomedical research: an internally self-consistent universe with little contact with medical reality? Nature Rev. Drug Discov. 2, 151–154 (2003).
Ruffolo, R. R. Why has R&D productivity declined in the pharmaceutical industry? Expert Opin. Drug Discov. 1 99–102 (2006).
Le Fanu, J. The Rise and Fall of Modern Medicine (Little Brown, London, 1999).
Pisano, G. Science Business: The Promise, the Reality, and the Future of Biotech. (Harvard Business School Press, Boston, 2006).
Young, M. P. Prediction v Attrition. Drug Discovery World website [online], (2008).
Hopkins, A. L., Mason, J. S. & Overington, J. P. Can we rationally design promiscuous drugs? Curr. Opin. Struct. Biol. 16, 127–136 (2006).
Tollman, P., Morieux, Y., Murphy, J. K. & Schulze, U. Identifying R&D outliers. Nature Rev. Drug Discov. 10, 653–654 (2011).
Ford, E. S. et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N. Engl. J. Med. 356, 2388–2398 (2007).
Lichtenberg, F. The impact of drug launches on longevity: evidence from longitudinal disease-level data from 52 countries, 1982–2001. Int. J. Health Care Finance Econ. 5, 47–73 (2005).
Schnee, J. E. R&D strategy in the U.S. pharmaceutical industry. Res. Policy 8, 364–382 (1979).
Hopkins, A. L. & Groom, C. R. The druggable genome. Nature Rev. Drug Discov. 1, 727–730 (2002).
Russ, A. P. & Lampel, S. The druggable genome: an update. Drug Discov. Today 10, 1607–1610 (2005).
Overington, J. P., Al-Lazikani, B. & Hopkins, A. L. How many drug targets are there? Nature Rev. Drug Discov. 5, 993–996 (2006).
Roth, B. L., Sheffer, D. L. & Kroeze, W. K. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nature Rev. Drug Discov. 3, 353–359 (2004).
Wurtman, R. J. & Bettiker, R. L. The slowing of treatment discovery, 1965–1995. Nature Med. 1, 1122–1125 (1995).
Healy, D. The Psychopharmacologists: Volume 2 93–118 (Hodder Arnold, London, 1999).
Healy, D. The Psychopharmacologists: Volume 2 259–264 (Hodder Arnold, London, 1999).
Healy, D. The Antidepressant Era (Harvard University Press, Cambridge, Massachusetts, 1997).
Weatherall, M. An end to the search for new drugs? Nature 296, 387–390 (1982).
Richard, J. & Wurtman, M. D. What went right: why is HIV a treatable infection? Nature Med. 3, 714–717 (1997).
[No authors listed.] A dearth of new drugs. Nature 283, 609 (1980).
Persson, C. G., Erjefält, J. S., Uller, L., Andersson, M. & Greiff, L. Unbalanced research. Trends Pharmacol. Sci. 22, 538–541 (2001).
Ainsworth, C. Networking for new drugs. Nature Med. 17, 1166–1168 (2011).
Denome, S. A., Elf, P. K., Henderson, T. A., Nelson, D. E. & Young, K. D. Escherichia coli mutants lacking all possible combinations of eight penicillin binding proteins: viability, characteristics, and implications for peptidoglycan synthesis. J. Bacteriol. 181, 3981–3993 (1999).
Keith, C. T., Borisy, A. A. & Stockwell, B. R. Multicomponent therapeutics for networked systems. Nature Rev. Drug Discov. 4, 71–78 (2005).
Lombardino, J. G. & Lowe, J. A. The role of the medicinal chemist in drug discovery — then and now. Nature Rev. Drug Discov. 3, 853–862 (2004).
Swinney, D. C. & Anthony, J. How were new medicines discovered? Nature Rev. Drug Discov. 10, 507–519 (2011).
Reichert, J. M. Probabilities of success for antibody therapeutics. mAbs 1, 387–389 (2009).
Steward, F. & Wibberly, G. Drug innovation — what's slowing it down? Nature 284, 118–120 (1980).
Collins, F. S. Medical and societal consequences of the Human Genome Project. N. Engl. J. Med. 341, 28–37 (1999).
Rees, J. Post-genome integrative biology: so that's what they call clinical science. Clin. Med. 1, 393–400 (2001).
Grove, A. Rethinking clinical trials. Science 333, 1679 (2011).
O'Shaughnessy, J. et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N. Engl. J. Med. 364, 205–214 (2011).
O'Shaughnessy, J. et al. A randomized Phase III study of iniparib (BSI-201) in combination with gemcitabine/carboplatin (G/C) in metastatic triple-negative breast cancer (TNBC). J. Clin. Oncol. 29, Abstr. 1007 (2011).
Guha, M. PARP inhibitors stumble in breast cancer. Nature Biotech. 29, 373–374 (2011).
Soda, M. et al. Identification of the transforming EML4–ALK fusion gene in non-small-cell lung cancer. Nature 448, 561–566 (2007).
Chapman, P. B. et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 364, 2507–2516 (2011).
[No authors listed.] Regulatory watch: leading hedgehog inhibitor submitted for approval as skin cancer drug. Nature Rev. Drug Discov. 10, 802–803 (2011).
DeMonaco, H. J., Ali, A. & von Hippel, E. The major role of clinicians in the discovery of off-label drug therapies. Pharmacotherapy 26, 323–332 (2006).
Mathieu, M. P. (ed.) Parexel's Bio/Pharmaceutical R&D Statistical Sourcebook 2010/2011 163–261 (Barnett International, Needham, Massachusetts, 2010).
Marshall, G. et al. Streptomycin treatment of pulmonary tuberculosis. BMJ 30, 769–782 (1948).
MacNeil, J. S. H. Changes in the characteristics of approved New Drug Applications for antihypertensives. Thesis, Massachusetts Institute of Technology (2007).
Lin, H. S. Changes in the characteristics of new drug applications for the treatment and prevention of diabetes mellitus. Thesis, Massachusetts Institute of Technology (2007).
Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian simvastatin survival study (4S). Lancet 344, 1383–1389 (1994).
Munos, B. How to avert biopharma's R&D crisis. In Vivo 29, 2011800050 (2011).
Macarron, R. et al. Impact of high-throughput screening in biomedical research. Nature Rev. Drug Discov. 10, 188–195 (2011).
Bohacek, R. S., McMartin, C. & Guida, W. C. The art and practice of structure-based drug design: a molecular modeling perspective. Med. Res. Rev. 16, 3–50 (1996).
Brown, D. Future pathways for combinatorial chemistry. Mol. Divers. 2, 217–222 (1996).
Lipinski, C. A., Lombardo, F., Dominy, B. W. & Feeney, P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 46, 3–26 (2001).
Gleeson, M. P., Hersey, A., Montanari, D. & Overington, J. Probing the links between in vitro potency, ADMET and physicochemical parameters. Nature Rev. Drug Discov. 10, 197–208 (2011).
Kay, J. Obliquity: Why our goals are best achieved indirectly (Profile Books, London, 2010).
Watts, D. J. & Strogatz, S. H. Collective dynamics of 'small-world' networks. Nature 393, 440–442 (1998).
Pharmaceutical Research and Manufacturers of America. Pharmaceutical Industry Profile 2011. PhRMA website [online], (Washington DC, PhRMA, April 2011).
Congress of the United States: Congressional Budget Office. Research and Development in the Pharmaceutical Industry. Congressional Budget Office (CBO) website [online], (October 2006).
Acknowledgements
W. Bains, T. Curtis, B. Charlton, M. Young, O. Imasogie, G. Porges, and B. Munos were generous with their time and ideas during various stages in the genesis of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Jack Scannell, Alex Blanckley and Helen Boldon are employees of Sanford C. Bernstein and declare the following interests: accounts over which Bernstein and/or their affiliates exercise investment discretion own more than 1% of the outstanding common stock of the following companies: Shire PLC. Bernstein currently makes a market in the following companies: Shire PLC. One or more of the officers, directors or employees of Sanford C. Bernstein & Co. LLC, Sanford C. Bernstein Limited, Sanford C. Bernstein (Hong Kong) Limited, Sanford C. Bernstein (business registration number 53193989L), a unit of AllianceBernstein (Singapore) Ltd., which is a licensed entity under the Securities and Futures Act and registered with Company Registration No. 199703364C, and/or their affiliates may at any time hold, increase or decrease positions in securities of any company mentioned herein. Bernstein or its affiliates may provide investment management or other services to the pension or profit sharing plans, or employees of any company mentioned herein, and may give advice to others as to investments in such companies. These entities may effect transactions that are similar to or different from those recommended herein.
Brian Warrington is a co-founder of BB consultants Ltd and provides advice to Phoenix IP Ventures.
Supplementary information
Supplementary information S1 (table)
New drug and cost estimates (XLS 123 kb)
Supplementary information S2 (Box)
Searching chemical space (PDF 219 kb)
Related links
Rights and permissions
About this article
Cite this article
Scannell, J., Blanckley, A., Boldon, H. et al. Diagnosing the decline in pharmaceutical R&D efficiency. Nat Rev Drug Discov 11, 191–200 (2012). https://doi.org/10.1038/nrd3681
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd3681
This article is cited by
-
Prediction of the active compounds and mechanism of Biochanin A in the treatment of Legg-Calvé-Perthes disease based on network pharmacology and molecular docking
BMC Complementary Medicine and Therapies (2024)
-
Drug design on quantum computers
Nature Physics (2024)
-
Albendazole inhibits colon cancer progression and therapy resistance by targeting ubiquitin ligase RNF20
British Journal of Cancer (2024)
-
Diagnosing the declining industry sponsorship in clinical research
Scientometrics (2024)
-
The Role of Genetics in Advancing Cardiometabolic Drug Development
Current Atherosclerosis Reports (2024)