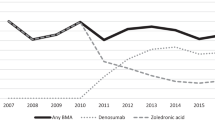
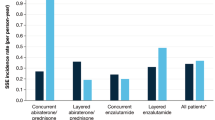
Key Points
-
Metastatic castration-resistant prostate cancer (mCRPC) is associated with complications in bone known as skeletal-related events (SREs) that are associated with decreased quality of life and increased mortality
-
Androgen-deprivation therapy is used to treat advanced-stage prostate cancer and leads to loss of bone mineral density (BMD) and increases the risk of fracture
-
Osteoclast-targeted agents including the bisphosphonate zoledronic acid and the monoclonal antibody denosumab are used to reduce the incidence of SREs and to decrease loss of BMD
-
A number of recently approved agents including androgen pathway inhibitors abiraterone and enzalutamide and the radiopharmaceutical radium-223 have been shown to increase survival and to decrease skeletal morbidity in mCRPC
-
Cabozantinib, a multi-targeted tyrosine kinase inhibitor, is an agent in development that has demonstrated promising activity in CRPC metastatic to bone
Abstract
Advanced-stage prostate cancer is associated with skeletal complications related to metastatic disease and its treatment. On the one hand, metastatic disease to bone is commonly associated with skeletal-related events (SREs); on the other hand, treatment with androgen-deprivation therapy (ADT) leads to loss in bone mineral density (BMD) and increased risk of fracture. Despite osteoblastic appearance on radiography, bone metastases from prostate cancer are associated with increased osteoblast and osteoclast activity providing the rationale for treatment with osteoclast-targeted agents. The bisphosphonate zoledronic acid and the monoclonal antibody denosumab reduce the incidence of SREs in metastatic castration-resistant prostate cancer (mCRPC). A number of agents prevent loss of BMD associated with ADT, but only denosumab is approved to reduce fractures in patients with non-metastatic prostate cancer. Another recently approved agent—radium-223—improves survival and delays SREs in mCRPC. The inhibitors of androgen receptor signalling, abiraterone and enzalutamide, improve survival in mCRPC and delay SREs, although the latter is likely related to control of disease rather than a direct effect on bone. Finally, the tyrosine kinase inhibitor cabozantinib shows promising activity in bone metastases from mCRPC. This Review addresses the skeletal morbidity associated with prostate cancer and the therapeutic options that exist to treat it.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
11 November 2014
In the version of this manuscript originally published online and in print, some data in Table 1 were incorrect. These errors have now been corrected in the online HTML and PDF versions of the manuscript.
References
Siegel, R., Naishadham, D. & Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 63, 11–30 (2013).
Jacobs, S. C. Spread of prostatic cancer to bone. Urology 21, 337–344 (1983).
Scher, H. I., Morris, M. J., Kelly, W. K., Schwartz, L. H. & Heller, G. Prostate cancer clinical trial end points: “RECIST”ing a step backwards. Clin. Cancer Res. 11, 5223–5232 (2005).
Coleman, R. E. Skeletal complications of malignancy. Cancer 80, 1588–1594 (1997).
DePuy, V. et al. Effects of skeletal morbidities on longitudinal patient-reported outcomes and survival in patients with metastatic prostate cancer. Support Care Cancer 15, 869–876 (2007).
Oefelein, M. G., Ricchiuti, V., Conrad, W. & Resnick, M. I. Skeletal fractures negatively correlate with overall survival in men with prostate cancer. J. Urol. 168, 1005–1007 (2002).
Alibhai, S. M. et al. Fracture types and risk factors in men with prostate cancer on androgen deprivation therapy: a matched cohort study of 19,079 men. J. Urol. 184, 918–923 (2010).
Manolagas, S. C. & Jilka, R. L. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N. Engl. J. Med. 332, 305–311 (1995).
Lacey, D. L. et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93, 165–176 (1998).
Yasuda, H. et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl Acad. Sci. USA 95, 3597–3602 (1998).
Kong, Y. Y. et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397, 315–323 (1999).
Hsu, H. et al. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc. Natl Acad. Sci. USA 96, 3540–3545 (1999).
Li, J. et al. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc. Natl Acad. Sci. USA 97, 1566–1571 (2000).
Simonet, W. S. et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89, 309–319 (1997).
Yasuda, H. et al. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology 139, 1329–1337 (1998).
Keller, E. T. & Brown, J. Prostate cancer bone metastases promote both osteolytic and osteoblastic activity. J. Cell. Biochem. 91, 718–729 (2004).
Guise, T. A. Molecular mechanisms of osteolytic bone metastases. Cancer 88, 2892–2898 (2000).
Saad, F. Bone-directed treatments for prostate cancer. Hematol. Oncol. Clin. North Am. 20, 947–963 (2006).
Snedecor, S. J., Carter, J. A., Kaura, S. & Botteman, M. F. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a cost-effectiveness analysis. J. Med. Econ. 16, 19–29 (2013).
Berruti, A. et al. Predictive factors for skeletal complications in hormone-refractory prostate cancer patients with metastatic bone disease. Br. J. Cancer 93, 633–638 (2005).
Tchekmedyian, N. S., Chen, Y. M. & Saad, F. Disease progression increases the risk of skeletal-related events in patients with bone metastases from castration-resistant prostate cancer, lung cancer, or other solid tumors. Cancer Invest. 28, 849–855 (2010).
Saad, F. et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J. Natl Cancer Inst. 96, 879–882 (2004).
Saad, F., Segal, S. & Eastham, J. Prostate-specific antigen kinetics and outcomes in patients with bone metastases from castration-resistant prostate cancer treated with or without zoledronic acid. Eur. Urol. 65, 146–153 (2014).
Brown, J. E. et al. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J. Natl Cancer Inst. 97, 59–69 (2005).
Lipton, A. et al. Skeletal-related events and clinical outcomes in patients with bone metastases and normal levels of osteolysis: exploratory analyses. Clin. Oncol. (R. Coll. Radiol.) 25, 217–226 (2013).
Smith, M. R. et al. Predictors of skeletal complications in men with hormone-refractory metastatic prostate cancer. Urology 70, 315–319 (2007).
de la Piedra, C. et al. Usefulness of bone turnover markers as predictors of mortality risk, disease progression and skeletal-related events appearance in patients with prostate cancer with bone metastases following treatment with zoledronic acid: TUGAMO study. Br. J. Cancer 108, 2565–2572 (2013).
Lipton, A. et al. Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer 113, 193–201 (2008).
Stoch, S. A. et al. Bone loss in men with prostate cancer treated with gonadotropin-releasing hormone agonists. J. Clin. Endocrinol. Metab. 86, 2787–2791 (2001).
Lee, H., McGovern, K., Finkelstein, J. S. & Smith, M. R. Changes in bone mineral density and body composition during initial and long-term gonadotropin-releasing hormone agonist treatment for prostate carcinoma. Cancer 104, 1633–1637 (2005).
Diamond, T. H., Higano, C. S., Smith, M. R., Guise, T. A. & Singer, F. R. Osteoporosis in men with prostate carcinoma receiving androgen-deprivation therapy: recommendations for diagnosis and therapies. Cancer 100, 892–899 (2004).
Shahinian, V. B., Kuo, Y. F., Freeman, J. L. & Goodwin, J. S. Risk of fracture after androgen deprivation for prostate cancer. N. Engl. J. Med. 352, 154–164 (2005).
Smith, M. R. et al. Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J. Clin. Oncol. 23, 7897–7903 (2005).
López, A. M. et al. Fracture risk in patients with prostate cancer on androgen deprivation therapy. Osteoporos. Int. 16, 707–711 (2005).
Beebe-Dimmer, J. L. et al. Timing of androgen deprivation therapy use and fracture risk among elderly men with prostate cancer in the United States. Pharmacoepidemiol. Drug Saf. 21, 70–78 (2012).
LeBlanc, E. S. et al. The effects of serum testosterone, estradiol, and sex hormone binding globulin levels on fracture risk in older men. J. Clin. Endocrinol. Metab. 94, 3337–3346 (2009).
Novara, G., Galfano, A., Secco, S., Ficarra, V. & Artibani, W. Impact of surgical and medical castration on serum testosterone level in prostate cancer patients. Urol. Int. 82, 249–255 (2009).
Vidal, O., Kindblom, L. G. & Ohlsson, C. Expression and localization of estrogen receptor-beta in murine and human bone. J. Bone Miner. Res. 14, 923–929 (1999).
[No authors listed] Management of osteoporosis in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause 17, 25–54 (2010).
Smith, M. R., Fallon, M. A., Lee, H. & Finkelstein, J. S. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J. Clin. Endocrinol. Metab. 89, 3841–3846 (2004).
Smith, M. R. et al. Toremifene to reduce fracture risk in men receiving androgen deprivation therapy for prostate cancer. J. Urol. 189 (Suppl. 1), S45–S50 (2013).
Lin, J. H. Bisphosphonates: a review of their pharmacokinetic properties. Bone 18, 75–85 (1996).
Saad, F. et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J. Natl Cancer Inst. 94, 1458–1468 (2002).
Rosen, L. S. et al. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J. 7, 377–387 (2001).
Neville-Webbe, H. L. & Coleman, R. E. Bisphosphonates and RANK ligand inhibitors for the treatment and prevention of metastatic bone disease. Eur. J. Cancer 46, 1211–1222 (2010).
Michaelson, M. D. et al. Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J. Clin. Oncol. 25, 1038–1042 (2007).
Kachnic, L. A. et al. RTOG 0518: randomized phase III trial to evaluate zoledronic acid for prevention of osteoporosis and associated fractures in prostate cancer patients. Prostate Cancer Prostatic Dis. 16, 382–386 (2013).
Wadhwa, V. K., Weston, R. & Parr, N. J. Frequency of zoledronic acid to prevent further bone loss in osteoporotic patients undergoing androgen deprivation therapy for prostate cancer. BJU Int. 105, 1082–1088 (2010).
Smith, M. R. et al. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J. Urol. 169, 2008–2012 (2003).
Klotz, L. H. et al. A phase 3, double-blind, randomised, parallel-group, placebo-controlled study of oral weekly alendronate for the prevention of androgen deprivation bone loss in nonmetastatic prostate cancer: the Cancer and Osteoporosis Research with Alendronate and Leuprolide (CORAL) study. Eur. Urol. 63, 927–935 (2013).
Greenspan, S. L. et al. Skeletal health after continuation, withdrawal, or delay of alendronate in men with prostate cancer undergoing androgen-deprivation therapy. J. Clin. Oncol. 26, 4426–4434 (2008).
Smith, M. R. et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N. Engl. J. Med. 345, 948–955 (2001).
Morabito, N. et al. Neridronate prevents bone loss in patients receiving androgen deprivation therapy for prostate cancer. J. Bone Miner. Res. 19, 1766–1770 (2004).
Dearnaley, D. P., Mason, M. D., Parmar, M. K., Sanders, K. & Sydes, M. R. Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: long-term overall survival results from the MRC PR04 and PR05 randomised controlled trials. Lancet Oncol. 10, 872–876 (2009).
Mason, M. D. et al. Oral sodium clodronate for nonmetastatic prostate cancer--results of a randomized double-blind placebo-controlled trial: Medical Research Council PR04 (ISRCTN61384873). J. Natl Cancer Inst. 99, 765–776 (2007).
Dearnaley, D. P. et al. A double-blind, placebo-controlled, randomized trial of oral sodium clodronate for metastatic prostate cancer (MRC PR05 Trial). J. Natl Cancer Inst. 95, 1300–1311 (2003).
Small, E. J., Smith, M. R., Seaman, J. J., Petrone, S. & Kowalski, M. O. Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J. Clin. Oncol. 21, 4277–4284 (2003).
Smith, M. R. et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J. Clin. Oncol. 23, 2918–2925 (2005).
Wirth, M. et al. Prevention of bone metastases in patients with high-risk nonmetastatic prostate cancer treated with zoledronic acid: efficacy and safety results of the Zometa European Study (ZEUS). Eur. Urol. http://dx.doi.org/10.1016/j.eururo.2014.02.014 (2014).
Smith, M. R. et al. Effects of denosumab on bone mineral density in men receiving androgen deprivation therapy for prostate cancer. J. Urol. 182, 2670–2675 (2009).
US National Library of Medicine. ClinicalTrials.gov[online], (2013).
US Food and Drug Administration. Denosumab (prolia) [online], (2011).
Jones, D. H. et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature 440, 692–696 (2006).
Smith, M. R. et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet 379, 39–46 (2012).
Smith, M. R. et al. Denosumab and bone metastasis-free survival in men with nonmetastatic castration-resistant prostate cancer: exploratory analyses by baseline prostate-specific antigen doubling time. J. Clin. Oncol. 30, 3800–3806 (2013).
Fizazi, K. et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 377, 813–822 (2011).
US Food and Drug Administration. Denosumab [online], (2010).
Robinson, R. G. et al. Strontium-89: treatment results and kinetics in patients with painful metastatic prostate and breast cancer in bone. Radiographics 9, 271–281 (1989).
Bauman, G., Charette, M., Reid, R. & Sathya, J. Radiopharmaceuticals for the palliation of painful bone metastasis-a systemic review. Radiother. Oncol. 75, 258–270 (2005).
Henriksen, G., Fisher, D. R., Roeske, J. C., Bruland, O. S. & Larsen, R. H. Targeting of osseous sites with alpha-emitting 223Ra: comparison with the beta-emitter 89Sr in mice. J. Nucl. Med. 44, 252–259 (2003).
Tannock, I. F. et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 351, 1502–1512 (2004).
Tu, S. M. et al. Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate: a randomised phase II trial. Lancet 357, 336–341 (2001).
James, N. D. et al. Clinical outcomes in patients with castrate-refractory prostate cancer (CRPC) metastatic to bone randomized in the factorial TRAPEZE trial to docetaxel (D) with strontium-89 (Sr89), zoledronic acid (ZA), neither, or both (ISRCTN 12808747) [abstract]. J. Clin. Oncol. 31 (Suppl.), LBA5000 (2013).
Bruland, Ø. S., Nilsson, S., Fisher, D. R. & Larsen, R. H. High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: adjuvant or alternative to conventional modalities? Clin. Cancer Res. 12, 6250s–6257s (2006).
Parker, C. et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 369, 213–223 (2013).
US Food and Drug Administration. Radium Ra 223 dichloride [online], (2013).
Nelson, J. B. et al. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nat. Med. 1, 944–949 (1995).
Yin, J. J. et al. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc. Natl Acad. Sci. USA 100, 10954–10959 (2003).
Saad, F. & Lipton, A. SRC kinase inhibition: targeting bone metastases and tumor growth in prostate and breast cancer. Cancer Treat. Rev. 36, 177–184 (2010).
Tatarov, O. et al. SRC family kinase activity is up-regulated in hormone-refractory prostate cancer. Clin. Cancer Res. 15, 3540–3549 (2009).
Nelson, J. B. et al. Phase 3, randomized, placebo-controlled study of zibotentan (ZD4054) in patients with castration-resistant prostate cancer metastatic to bone. Cancer 118, 5709–5718 (2012).
Fizazi, K. S. et al. Phase III, randomized, placebo-controlled study of docetaxel in combination with zibotentan in patients with metastatic castration-resistant prostate cancer. J. Clin. Oncol. 31, 1740–1747 (2013).
Nelson, J. B. et al. Phase 3, randomized, controlled trial of atrasentan in patients with nonmetastatic, hormone-refractory prostate cancer. Cancer 113, 2478–2487 (2008).
Quinn, D. I. et al. Docetaxel and atrasentan versus docetaxel and placebo for men with advanced castration-resistant prostate cancer (SWOG S0421): a randomised phase 3 trial. Lancet Oncol. 14, 893–900 (2013).
Araujo, J. C. et al. Docetaxel and dasatinib or placebo in men with metastatic castration-resistant prostate cancer (READY): a randomised, double-blind phase 3 trial. Lancet Oncol. 14, 1307–1316 (2013).
de Bono, J. S. et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 364, 1995–2005 (2011).
Logothetis, C. J. et al. Effect of abiraterone acetate and prednisone compared with placebo and prednisone on pain control and skeletal-related events in patients with metastatic castration-resistant prostate cancer: exploratory analysis of data from the COU-AA-301 randomised trial. Lancet Oncol. 13, 1210–1217 (2012).
Ryan, C. J. et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 368, 138–148 (2013).
Saad, F. et al. Impact of concomitant bone-targeted therapies (BTT) on outcomes in metastatic castration-resistant prostate cancer (mCRPC) patients (pts) without prior chemotherapy (ctx) treated with abiraterone acetate (AA) or prednisone (P) [abstract]. J. Clin. Oncol. 31 (Suppl.), a5037 (2013).
Scher, H. I. et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 367, 1187–1197 (2012).
US National Library of Medicine. ClinicalTrials.gov[online], (2013).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
Smith, D. C. et al. Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. J. Clin. Oncol. 31, 412–419 (2013).
Dai, J. et al. Cabozantinib inhibits prostate cancer growth and prevents tumor-induced bone lesions. Clin. Cancer Res. 20, 617–630 (2014).
US National Library of Medicine. ClinicalTrials.gov[online], (2013).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
Epstein, J. et al. Postradiation osteonecrosis of the mandible: a long-term follow-up study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 83, 657–662 (1997).
Advisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws & American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J. Oral Maxillofac. Surg. 65, 369–376 (2007).
Pazianas, M., Miller, P., Blumentals, W. A., Bernal, M. & Kothawala, P. A review of the literature on osteonecrosis of the jaw in patients with osteoporosis treated with oral bisphosphonates: prevalence, risk factors, and clinical characteristics. Clin. Ther. 29, 1548–1558 (2007).
Bamias, A. et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J. Clin. Oncol. 23, 8580–8587 (2005).
Saad, F. et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann. Oncol. 23, 1341–1347 (2012).
Badros, A. et al. Osteonecrosis of the jaw in multiple myeloma patients: clinical features and risk factors. J. Clin. Oncol. 24, 945–952 (2006).
Ripamonti, C. I. et al. Decreased occurrence of osteonecrosis of the jaw after implementation of dental preventive measures in solid tumour patients with bone metastases treated with bisphosphonates. The experience of the National Cancer Institute of Milan. Ann. Oncol. 20, 137–145 (2009).
Dimopoulos, M. A. et al. Reduction of osteonecrosis of the jaw (ONJ) after implementation of preventive measures in patients with multiple myeloma treated with zoledronic acid. Ann. Oncol. 20, 117–120 (2009).
Perazella, M. A. & Markowitz, G. S. Bisphosphonate nephrotoxicity. Kidney Int. 74, 1385–1393 (2008).
Chang, J. T., Green, L. & Beitz, J. Renal failure with the use of zoledronic acid. N. Engl. J. Med. 349, 1676–1679 (2003).
Block, G. A., Bone, H. G., Fang, L., Lee, E. & Padhi, D. A single-dose study of denosumab in patients with various degrees of renal impairment. J. Bone Miner. Res. 27, 1471–1479 (2012).
Author information
Authors and Affiliations
Contributions
Both authors researched the data for the article, provided a substantial contribution to discussions of the content, wrote the article and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
F.S. is a consultant and receives honoraria from Novartis and Amgen. B.A.G. declares no competing interests.
Rights and permissions
About this article
Cite this article
Gartrell, B., Saad, F. Managing bone metastases and reducing skeletal related events in prostate cancer. Nat Rev Clin Oncol 11, 335–345 (2014). https://doi.org/10.1038/nrclinonc.2014.70
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2014.70
This article is cited by
-
Outcomes and Factors Associated with Completion of Radium-223 Therapy
Nuclear Medicine and Molecular Imaging (2022)
-
Acetylation of KLF5 maintains EMT and tumorigenicity to cause chemoresistant bone metastasis in prostate cancer
Nature Communications (2021)
-
Matching-Adjusted Indirect Comparison of Health-Related Quality of Life and Adverse Events of Apalutamide Versus Enzalutamide in Non-Metastatic Castration-Resistant Prostate Cancer
Advances in Therapy (2020)
-
Population Pharmacokinetics of Apalutamide and its Active Metabolite N-Desmethyl-Apalutamide in Healthy and Castration-Resistant Prostate Cancer Subjects
Clinical Pharmacokinetics (2020)
-
Engineering osteoblastic metastases to delineate the adaptive response of androgen-deprived prostate cancer in the bone metastatic microenvironment
Bone Research (2019)