Abstract
Background
In an era of multiple life-prolonging therapies for metastatic castration resistant prostate cancer (mCRPC), the optimal timing of initiation and duration of antiresorptive bone targeted therapy (BTT) to prevent skeletal related events (SREs) is unknown.
Methods
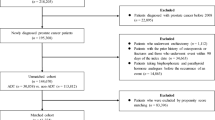
To assess practice patterns of BTT use and its associations with clinical outcomes in a high-volume center in the modern era of metastatic CRPC management, a retrospective cohort of patients treated for mCRPC with BM between 2007 and 2017 was identified from a single institutions clinical research database. Study endpoints included time from the diagnosis of CRPC to the onset of SRE or OS. Cox proportional hazards model assessed association of BTT use with time to first SRE and OS.
Results
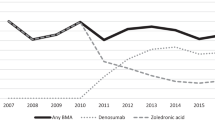
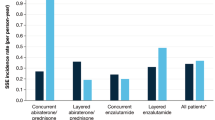
In total, 249 patients were identified; median follow-up was 7.7 (95%CI: 5.7–10.2) years. On multivariable analysis, patients with 4 or more BM at diagnosis of mCRPC who received BTT with abiraterone acetate or enzalutamide as first line therapy had a 42% reduced risk of developing an SRE (HR 0.58; 95%CI: 0.36–0.95) compared to those who never received BTT or received it in second line. No such effect was observed in patients with 1–3 BM. No OS difference was noted in patients who received BTT, whether with first line therapy or without. This study is limited by retrospective nature at a single institution.
Conclusions
Our hospital registry data indicate a potential benefit in terms of SRE prevention for early use of antiresorptive BTT in combination with life prolonging CRPC therapies for patients with CRPC and at least 4 BM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Hernandez RK, Wade SW, Reich A, Pirolli M, Liede A, Lyman GH. Incidence of bone metastases in patients with solid tumors: analysis of oncology electronic medical records in the United States. BMC Cancer. 2018;18:44-.
Schulman KL, Kohles J. Economic burden of metastatic bone disease in the U.S. Cancer. 2007;109:2334–42.
McKay R, Haider B, Duh MS, Valderrama A, Nakabayashi M, Fiorillo M, et al. Impact of symptomatic skeletal events on health-care resource utilization and quality of life among patients with castration-resistant prostate cancer and bone metastases. Prostate Cancer Prostatic Dis. 2017;20:276–82.
Smith M, Parker C, Saad F, Miller K, Tombal B, Ng QS, et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:408–19.
Saad F, Fleshner NE, So A, Le Lorier J, Perrault L, Poulin-Costello M, et al. The burden of symptomatic skeletal events in castrate-resistant prostate cancer patients with bone metastases at three Canadian uro-oncology centres. Can Urological Assoc J = J de l’Assoc des urologues du Can. 2018;12:370–6.
Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer. Inst. 2002;94:1458–68.
Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96:879–82.
Fizazi K, Carducci M, Smith M, Damiao R, Brown J, Karsh L, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–22.
Smith MR, Halabi S, Ryan CJ, Hussain A, Vogelzang N, Stadler W, et al. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (Alliance). J Clin Oncol. 2014;32:1143–50.
James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–77.
Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PFA, Sternberg CN, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–60.
Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33.
Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fossa SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23.
Oudard S. TROPIC: phase III trial of cabazitaxel for the treatment of metastatic castration-resistant prostate cancer. Future Oncol. 2011;7:497–506.
Francini E, Gray KP, Xie W, Shaw GK, Valenca L, Bernard B, et al. Time of metastatic disease presentation and volume of disease are prognostic for metastatic hormone sensitive prostate cancer (mHSPC). Prostate. 2018;78:889–95.
Macherey S, Monsef I, Jahn F, Jordan K, Yuen KK, Heidenreich A, Skoetz N. Bisphosphonates for advanced prostate cancer. Cochrane Database Syst Rev. 2017;12:CD006250. https://doi.org/10.1002/14651858.CD006250.pub2. PMID: 29278410; PMCID: PMC6486306..
Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630–42.
Sweeney CJ, Chen Y-H, Carducci M, Liu G, Jarrard DF, Eisenberger M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–46.
Logothetis CJ, Basch E, Molina A, Fizazi K, North SA, Chi KN, et al. Effect of abiraterone acetate and prednisone compared with placebo and prednisone on pain control and skeletal-related events in patients with metastatic castration-resistant prostate cancer: exploratory analysis of data from the COU-AA-301 randomised trial. Lancet Oncol. 2012;13:1210–7.
Fizazi K, Scher HI, Miller K, Basch E, Sternberg CN, Cella D, et al. Effect of enzalutamide on time to first skeletal-related event, pain, and quality of life in men with castration-resistant prostate cancer: results from the randomised, phase 3 AFFIRM trial. Lancet Oncol. 2014;15:1147–56.
Saad F, Shore N, Van Poppel H, Rathkopf DE, Smith MR, de Bono JS, et al. Impact of bone-targeted therapies in chemotherapy-naive metastatic castration-resistant prostate cancer patients treated with abiraterone acetate: post hoc analysis of study COU-AA-302. Eur Urol. 2015;68:570–7.
Scher HI, Fizazi K, Saad F, Taplin M-E, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97.
de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005.
Beer TM, Armstrong AJ, Rathkopf D, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in men with chemotherapy-naive metastatic castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol. 2017;71:151–4.
Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2012;368:138–48.
Smith MR, Saad F, Oudard S, Shore N, Fizazi K, Sieber P, et al. Denosumab and bone metastasis-free survival in men with nonmetastatic castration-resistant prostate cancer: exploratory analyses by baseline prostate-specific antigen doubling time. J Clin Oncol. 2013;31:3800–6.
Saad F, Brown JE, Van Poznak C, Ibrahim T, Stemmer SM, Stopeck AT, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol. 2012;23:1341–7.
Ripamonti CI, Maniezzo M, Campa T, Fagnoni E, Brunelli C, Saibene G, et al. Decreased occurrence of osteonecrosis of the jaw after implementation of dental preventive measures in solid tumour patients with bone metastases treated with bisphosphonates. The experience of the National Cancer Institute of Milan. Annals of oncology: official journal of the European Society for. Med Oncol. 2009;20:137–45.
Smith MR, Egerdie B, Hernández Toriz N, Feldman R, Tammela TLJ, Saad F, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745–55.
Sartor O, Coleman R, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol. 2014;15:738–46.
Loriot Y, Miller K, Sternberg CN, Fizazi K, De Bono JS, Chowdhury S, et al. Effect of enzalutamide on health-related quality of life, pain, and skeletal-related events in asymptomatic and minimally symptomatic, chemotherapy-naive patients with metastatic castration-resistant prostate cancer (PREVAIL): results from a randomised, phase 3 trial. Lancet Oncol. 2015;16:509–21.
Tombal BF, Loriot Y, Saad F, McDermott RS, Elliott T, Rodriguez-Vida A., et al. Decreased fracture rate by mandating bone-protecting agents in the EORTC 1333/PEACE III trial comparing enzalutamide and Ra223 versus enzalutamide alone: an interim safety analysis. J Clin Oncol.2019;37 Suppl 15:5007.
Himelstein AL, Foster JC, Khatcheressian JL, Roberts JD, Seisler DK, Novotny PJ, et al. Effect of longer-interval vs standard dosing of zoledronic acid on skeletal events in patients with bone metastases: a randomized clinical trial. JAMA. 2017;317:48–58.
Clemons MJ, Ong M, Stober C, Ernst DS, Booth CM, Canil CM.et al. A randomized trial comparing four-weekly versus 12-weekly administration of bone-targeted agents (denosumab, zoledronate, or pamidronate) in patients with bone metastases from either breast or castration-resistant prostate cancer. J Clin Oncol.2019;37 Suppl 15:11501-.
Acknowledgements
We thank Kevin Pels, PhD of Dana-Farber Cancer Institute for manuscript editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
BM: discloses payment for consulting with Bayer, Astellas, Astra Zeneca, Seattle Genetics, Exelixis, Nektar, Pfizer, Janssen, Genentech, and EMD Serono. He received research support to Dana Farber Cancer Institute (DFCI) from Bristol Myers Squibb, Calithera, Exelixis, Seattle Genetics. C.S.: discloses a consulting or advisory at Sanofi, Janssen, Astellas Pharma, Bayer, Genentech, AstraZeneca, Pfizer, Lilly Celgene Research Funding: Janssen Biotech (Inst), Astellas Pharma (Inst), Sanofi (Inst), Bayer (Inst), Sotio (Inst), Dendreon (Inst). He has patents, Royalties, Other Intellectual Property for Pathenolide (Indiana University): dimethylaminoparthenolide (Leuchemix); Exelixis: Abiraterone plus cabozantinib combination. He owns stock in Leuchemix; LZ, KG, EF, GS, and CE report no conflict of interest.
Ethics approval
The study was performed in accordance with the Declaration of Helsinki; all patients were consented for their clinical data collection and the protocol was approved by DF/HCC IRB.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
McGregor, B., Zhang, L., Gray, K.P. et al. Bone targeted therapy and skeletal related events in the era of enzalutamide and abiraterone acetate for castration resistant prostate cancer with bone metastases. Prostate Cancer Prostatic Dis 24, 341–348 (2021). https://doi.org/10.1038/s41391-020-00280-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-020-00280-6