Abstract
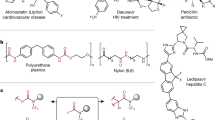
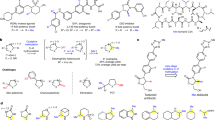
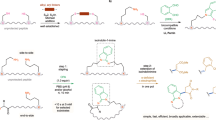
This protocol presents a detailed description of the synthesis of N-methylated cyclic peptides. N-methylation is a powerful technique to modulate the physicochemical properties of peptides by introducing one or more methyl groups into the peptidic amide bonds. Together with peptide cyclization, this procedure confers unprecedented pharmacokinetic properties to the peptides, including metabolic stability, membrane permeability and even oral bioavailability. Here we describe two simplified methods of N-methylation of linear peptides on solid supports, which can be performed in less than 2 h and are applicable to any amino acid. Finally, we also describe two methods of peptide cyclization, which can be used to obtain the N-methylated cyclic peptide and which are not limited to specific peptide sequences. With this protocol, multiply N-methylated cyclic peptides can be synthesized in as little as 4–5 d.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Gilon, C., Dechantsreiter, M.A., Burkhart, F., Friedler, A. & Kessler, H. in Houben-Weyl Methods of Organic Chemistry, Vol. E22c (eds. Goodman, M., Felix, A., Moroder, L. & Tonolio, C.) 215–291 (Georg Thieme Verlag, 2002).
Hamman, J.H., Enslin, G.M. & Kotze, A.F. Oral delivery of peptide drugs: barriers and developments. Biodrugs 19, 165–177 (2005).
Kessler, H. Peptide Conformations. 19. Conformation and biological activity of cyclic-peptides. Angew. Chem. Int. Ed. 21, 512–523 (1982).
Hess, S. et al. Backbone cyclic peptidomimetic melanocortin-4 receptor agonist as a novel orally administrated drug lead for treating obesity. J. Med. Chem. 51, 1026–1034 (2008).
Shemyakin, M.M., Ovchinnikov, Y.A. & Ivanov, V.T. Topochemical investigation of peptide systems. Angew. Chem. Int. Ed. Engl. 8, 492–499 (1969).
Goodman, M. & Chorev, M. Concept of linear modified retro-peptide structures. Acc. Chem. Res. 12, 1–7 (1979).
Giannis, A. Peptidomimetics for receptor ligands-discovery, development, and medical perspectives. Angew. Chem. Int. Ed. 32, 1244–1267 (1993).
Lee, H.K. et al. Anticonvulsant Met-enkephalin analogues containing backbone spacers reveal alternative non-opioid signaling in the brain. ACS Chem. Biol. 4, 659–671 (2009).
Simon, R.J. et al. Peptoids—a modular approach to drug discovery. Proc. Natl. Acad. Sci. USA 89, 9367–9371 (1992).
Kessler, H. Peptoids—a new approach to the development of pharmaceuticals. Angew. Chem. Int. Ed. Engl. 32, 543–544 (1993).
White, C.J. & Yudin, A.K. Contemporary strategies for peptide macrocyclization. Nat. Chem. 3, 509–524 (2011).
Rothbard, J.B. et al. Conjugation of arginine oligomers to cyclosporin A facilitates topical delivery and inhibition of inflammation. Nat. Med. 6, 1253–1257 (2000).
Wender, P.A. et al. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proc. Natl. Acad. Sci. USA 97, 13003–13008 (2000).
Richard, J.P. et al. Cell-penetrating peptides—a re-evaluation of the mechanism of cellular uptake. J. Biol. Chem. 278, 585–590 (2003).
Jones, S.W. et al. Characterisation of cell-penetrating peptide-mediated peptide delivery. Br. J. Pharmacol. 145, 1093–1102 (2005).
Borel, J.F., Feurer, C., Gubler, H.U. & Stahelin, H. Biological effects of Cyclosporin-a—new antilymphocytic agent. Agents Actions 6, 468–475 (1976).
Pettit, G.R. et al. Isolation of Dolastatins 10–15 from the marine mollusk Dolabella auricularia. Tetrahedron 49, 9151–9170 (1993).
Cruz, L.J. et al. IB-01212, a new cytotoxic cyclodepsipeptide isolated from the marine fungus Clonostachys sp. ESNA-A009. J. Org. Chem. 71, 3335–3338 (2006).
Plaza, A. et al. Mutremdamide A and koshikamides C-H, peptide inhibitors of HIV-1 entry from different Theonella species. J. Org. Chem. 75, 4344–4355 (2010).
Lipinski, C.A., Lombardo, F., Dominy, B.W. & Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 46, 3–26 (2001).
Sugano, H., Higaki, K. & Miyoshi, M. Synthesis and biological-activity of peptides related to eledoisin. 1. Hexapeptide amides containing alpha-hydroxy acids. Bull. Chem. Soc. Japan 46, 226–230 (1973).
Dechantsreiter, M.A. et al. N-methylated cyclic RGD peptides as highly active and selectiveα(v)β(3) integrin antagonists. J. Med. Chem. 42, 3033–3040 (1999).
Mas-Moruno, C., Rechenmacher, F. & Kessler, H. Cilengitide: the first anti-angiogenic small molecule drug candidate design, synthesis and clinical evaluation. Anticancer Agents Med. Chem. 10, 753–768 (2011).
Haubner, R., Finsinger, D. & Kessler, H. Stereoisomeric peptide libraries and peptidomimetics for designing selective inhibitors of the αvβ3 integrin for a new cancer therapy. Angew. Chem. Int. Ed. Engl. 36, 1374–1389 (1997).
Gordon, D.J., Tappe, R. & Meredith, S.C. Design and characterization of a membrane permeable N-methyl amino acid-containing peptide that inhibits A β(1–40) fibrillogenesis. J. Pept. Res. 60, 37–55 (2002).
Kessler, H. Detection of intramolecular mobility by NMR spectroscopy. 13. Detection of hindered rotation and inversion by NMR spectroscopy. Angew. Chem. Int. Ed. 9, 219–235 (1970).
Chatterjee, J., Gilon, C., Hoffman, A. & Kessler, H. N-methylation of peptides: a new perspective in medicinal chemistry. Acc. Chem. Res. 41, 1331–1342 (2008).
Ovadia, O. et al. Improvement of drug-like properties of peptides: the somatostatin paradigm. Expert Opin. Drug Discov. 5, 655–671 (2010).
Chatterjee, J., Mierke, D.F. & Kessler, H. Conformational preference and potential templates of N-methylated cyclic pentaalanine peptides. Chem. Eur. J. 14, 1508–1517 (2008).
Laufer, B., Chatterjee, J., Frank, A.O. & Kessler, H. Can N-methylated amino acids serve as substitutes for prolines in conformational design of cyclic pentapeptides? J. Pept. Sci. 15, 141–146 (2009).
Chatterjee, J. et al. Multiple N-methylation by a designed approach enhances receptor selectivity. J. Med. Chem. 50, 5878–5881 (2007).
Chatterjee, J. et al. N-Methylated sst2 selective somatostatin cyclic peptide analogue as potent candidate for treating neurogenic inflammation. ACS Med. Chem. Lett. 2, 509–514 (2011).
Doedens, L. et al. Multiple N-methylation of MT-II backbone amide bonds leads to melanocortin receptor subtype hMC1R selectivity: pharmacological and conformational studies. J. Am. Chem. Soc. 132, 8115–8128 (2010).
Biron, E. et al. Improving oral bioavailability of peptides by multiple N-methylation: somatostatin analogues. Angew. Chem. Int. Ed. 47, 2595–2599 (2008).
Ovadia, O. et al. The effect of multiple N-methylation on intestinal permeability of peptides. Mol. Pharmaceutics 8, 479–487 (2011).
Biron, E., Chatterjee, J. & Kessler, H. Optimized selective N-methylation of peptides on solid support. J. Pept. Sci. 12, 213–219 (2006).
Coin, I., Beyermann, M. & Bienert, M. Solid-phase peptide synthesis: from standard procedures to the synthesis of difficult sequences. Nat. Protoc. 2, 3247–3256 (2007).
Fukuyama, T., Jow, C.-K. & Cheung, M. 2- and 4-Nitrobenzenesulfonamides: exceptionally versatile means for preparation of secondary amines and protection of amines. Tetrahedron Lett. 36, 6373–6374 (1995).
Patgiri, A., Menzenski, M.Z., Mahon, A.B. & Arora, P.S. Solid-phase synthesis of short α-helices stabilized by the hydrogen bond surrogate approach. Nat. Protoc. 5, 1857–1865 (2010).
Kim, Y.W., Grossmann, T.N. & Verdine, G.L. Synthesis of all-hydrocarbon stapled α-helical peptides by ring-closing olefin metathesis. Nat. Protoc. 6, 761–771 (2011).
Malesevic, M., Strijowski, U., Bächle, D. & Sewald, N. An improved method for the solution cyclization of peptides under pseudo-high dilution conditions. J. Biotechnol. 112, 73–77 (2004).
Chatterjee, J., Mierke, D. & Kessler, H. N-methylated cyclic pentaalanine peptides as template structures. J. Am. Chem. Soc. 128, 15164–15172 (2006).
Acknowledgements
We thank M.M. Baksh of the Scripps Research Institute for his help in proofreading and editing the manuscript.
Author information
Authors and Affiliations
Contributions
J.C. and B.L. conducted the experiments as reported in the original papers and tested the protocol. H.K. supervised the overall project. J.C. wrote the manuscript, B.L. and H.K. edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Chatterjee, J., Laufer, B. & Kessler, H. Synthesis of N-methylated cyclic peptides. Nat Protoc 7, 432–444 (2012). https://doi.org/10.1038/nprot.2011.450
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2011.450
This article is cited by
-
An amide to thioamide substitution improves the permeability and bioavailability of macrocyclic peptides
Nature Communications (2023)
-
Bioactivity Screening of Hydrolysates From Brown Crab Processing Side Streams Fermented by Marine Pseudoalteromonas Strains
Waste and Biomass Valorization (2021)
-
Non-lipopeptide fungi-derived peptide antibiotics developed since 2000
Biotechnology Letters (2019)
-
Sustainable synthesis of N-methylated peptides in a continuous-flow fixed bed reactor
Journal of Flow Chemistry (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.