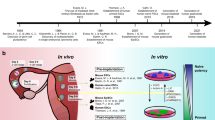
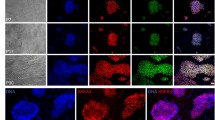
Abstract
The protocols described here are comprehensive instructions for deriving human embryonic stem (hES) cell lines in xeno-free conditions from cryopreserved embryos. Details are included for propagation, cryopreservation and characterization. Initial derivation is on feeder cells and is followed by adaptation to a feeder-free environment; competent technicians can perform these simplified methods easily. From derivation to cryopreservation of fully characterized initial stocks takes 3–4 months. These protocols served as the basis for standard operating procedures (SOPs), with both operational and technical components, that we set to meet good manufacturing practice (GMP) and UK regulatory body requirements for derivation of clinical-grade cells. As such, these SOPs are currently used in our current GMP compliant facility to derive hES cell lines ab initio, in an animal product–free environment; these lines are suitable for research and potentially for clinical use in cell therapy. So far, we have derived eight clinical-grade lines, which will be freely available to the scientific community after submission/accession to the UK Stem Cell Bank.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Thomson, J.C. et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998).
Salaway, T. & Ilic, D. Logistics of stem cell isolation, preparation and delivery for heart repair: concerns of clinicians, manufacturers, investors and public health. Regen. Med. 3, 83–91 (2008).
Ammann, A.J. et al. Acquired immunodeficiency in an infant: possible transmission by means of blood products. Lancet 321, 956–958 (1983).
Quint, W.G., Fetter, W.P., van Os, H.C. & Heijtink, R.A. Absence of hepatitis B virus (HBV) DNA in children born after exposure of their mothers to HBV during in vitro fertilization. J. Clin. Microbiol. 32, 1099–1100 (1994).
Llewelyn, C.A. et al. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet 363, 417–421 (2004).
Cobo, F. et al. Electron microscopy reveals the presence of viruses in mouse embryonic fibroblasts but neither in human embryonic fibroblasts nor in human mesenchymal cells used for hESC maintenance: toward an implementation of microbiological quality assurance program in stem cell banks. Cloning Stem Cells 10, 65–74 (2008).
Braude, P., Minger, S.L. & Warwick, R.M. Stem cell therapy: hope or hype? BMJ 330, 1159–1160 (2005).
Schwartz, S.D. et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet 379, 713–720 (2012).
Brindley, D. & Mason, C. Human embryonic stem cell therapy in the post-Geron era. Regen. Med. 7, 17–18 (2012).
Crook, J.M. et al. The generation of six clinical-grade human embryonic stem cell lines. Cell Stem Cell 1, 490–494 (2007).
Lei, T. et al. Xeno-free derivation and culture of human embryonic stem cells: current status, problems and challenges. Cell Res. 17, 682–688 (2007).
Valamehr, B., Tsutsui, H., Ho, C.M. & Wu, H. Developing defined culture systems for human pluripotent stem cells. Regen. Med. 6, 623–634 (2011).
Meng, G., Liu, S., Li, X., Krawetz, R. & Rancourt, D.E. Extracellular matrix isolated from foreskin fibroblasts supports long-term xeno-free human embryonic stem cell culture. Stem Cells Dev. 19, 547–556 (2010).
Ellerstrom, C. et al. Derivation of a xeno-free human embryonic stem cell line. Stem. Cells 24, 2170–2176 (2006).
Rajala, K. et al. A defined and xeno-free culture method enabling the establishment of clinical-grade human embryonic, induced pluripotent and adipose stem cells. PLoS ONE 5, e10246 (2010).
Ilic, D. et al. Derivation and feeder-free propagation of human embryonic stem cells under xeno-free conditions. Cytotherapy 14, 122–128 (2012).
Nakajima, F., Tokunaga, K. & Nakatsuji, N. Human leukocyte antigen matching estimations in a hypothetical bank of human embryonic stem cell lines in the Japanese population for use in cell transplantation therapy. Stem Cells 25, 983–985 (2007).
Taylor, C.J. et al. Banking on human embryonic stem cells: estimating the number of donor cell lines needed for HLA matching. Lancet 366, 2019–2025 (2005).
Taylor, C.J., Bolton, E.M. & Bradley, J.A. Immunological considerations for embryonic and induced pluripotent stem cell banking. Philos. Trans. R Soc. Lond. B Biol. Sci. 366, 2312–2322 (2011).
Hunt, C.J. Cryopreservation of human stem cells for clinical application: a review. Transfus. Med. Hemother. 38, 107–123 (2011).
Skottman, H., Narkilahti, S. & Hovatta, O. Challenges and approaches to the culture of pluripotent human embryonic stem cells. Regen. Med. 2, 265–273 (2007).
Pickering, S.J. et al. Preimplantation genetic diagnosis as a novel source of embryos for stem cell research. Reprod. Biomed. Online 7, 353–364 (2003).
Ström, S. et al. Mechanical isolation of the inner cell mass is effective in derivation of new human embryonic stem cell lines. Hum. Reprod. 22, 3051–3058 (2007).
Stephenson, E.L. & Braude, P.R. Derivation of the King's College London human embryonic stem cell lines. In Vitro Cell Dev. Biol. Anim. 46, 178–185 (2010).
Ström, S., Holm, F., Bergström, R., Strömberg, A.M. & Hovatta, O. Derivation of 30 human embryonic stem cell lines—improving the quality. In Vitro Cell Dev. Biol. Anim. 46, 337–344 (2010).
Bergström, R., Ström, S., Holm, F., Feki, A. & Hovatta, O. Xeno-free culture of human pluripotent stem cells. Methods Mol. Biol. 767, 125–136 (2011).
Ilic, D. Culture of human embryonic stem cells and the extracellular matrix microenvironment. Regen. Med. 1, 95–101 (2006).
Ilic, D. et al. Derivation of human embryonic stem cell lines from biopsied blastomeres on human feeders with minimal exposure to xenomaterials. Stem. Cells Dev. 18, 1343–1350 (2009).
Reubinoff, B.E., Pera, M.F., Vajta, G. & Trounson, A.O. Effective cryopreservation of human embryonic stem cells by the open pulled straw vitrification method. Hum. Reprod. 16, 2187–2194 (2001).
Zhou, C.Q., Mai, Q.Y., Li, T. & Zhuang, G.L. Cryopreservation of human embryonic stem cells by vitrification. Chin. Med. J. (Engl.) 117, 1050–1055 (2004).
Ilic, D., Genbacev, O. & Krtolica, A. Derivation of hESC from intact blastocysts. Curr. Protoc. Stem Cell Biol 1, 1A.2.1–1A.2.18 (2007).
Zhang, X. et al. Derivation of human embryonic stem cells from developing and arrested embryos. Stem Cells 24, 2669–2676 (2006).
Feki, A. et al. Derivation of the first Swiss human embryonic stem cell line from a single blastomere of an arrested four-cell stage embryo. Swiss Med. Wkly. 138, 540–550 (2008).
Klimanskaya, I., Chung, Y., Becker, S., Lu, S.J. & Lanza, R. Human embryonic stem cell lines derived from single blastomeres. Nature 444, 481–485 (2006).
Klimanskaya, I., Chung, Y., Becker, S., Lu, S.J. & Lanza, R. Derivation of human embryonic stem cells from single blastomeres. Nat. Protoc. 2, 1963–1972 (2007).
Chung, Y. et al. Human embryonic stem cell lines generated without embryo destruction. Cell Stem Cell 2, 113–117 (2008).
Giritharan, G., Ilic, D., Gormley, M. & Krtolica, A. Human embryonic stem cells derived from embryos at different stages of development share similar transcription profiles. PLoS ONE 6, e26570 (2011).
Ilic, D. et al. Effect of karyotype on successful human embryonic stem cell derivation. Stem Cells Dev. 19, 39–46 (2010).
Adewumi, O. et al. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat. Biotechnol. 25, 803–816 (2007).
Strulovici, Y., Leopold, P.L., O'Connor, T.P., Pergolizzi, R.G. & Crystal, R.G. Human embryonic stem cells and gene therapy. Mol. Ther. 15, 850–866 (2007).
Stephenson, E.L., Braude, P.R. & Mason, C. Proposal for a universal minimum information convention for the reporting on the derivation of human embryonic stem cell lines. Regen. Med. 1, 739–750 (2006).
Stephenson, E. et al. Safety paradigm: genetic evaluation of therapeutic grade human embryonic stem cells. J. R. Soc. Interface 7 (suppl. 6), S677–S688 (2010).
Acknowledgements
This work was supported by the Medical Research Council, UK (grants G0801061 and G0701172). We thank our collaborators at the Cytogenetics Department and the Clinical Transplantation Laboratory, Guy's Hospital, for genetic analyses and HLA typing; A. Huhn and P. Sharpe at the Dental Institute and C. Hobbs, Histology Manager at the Wolfson Centre for Age-Related Diseases, School of Biomedical Sciences, King's College London, for teratoma-related work; and J.-R. Fantino from Institut de Microbiologie de la Méditerranée, Centre National de la Recherche Scientifique, Marseille, for editing movies. We also want to thank Y. Khalaf, director of the Assisted Conception Unit of Guy's and St. Thomas' National Health Service (NHS) Foundation Trust, and his staff for supporting the research program. We are especially indebted to the patients who donated embryos.
Author information
Authors and Affiliations
Contributions
E.S., P.B. and D.I. designed the protocol, carried out the work, analyzed the results and prepared the manuscript. L.J. and C.M. designed the protocol, carried out the work and analyzed the results. V.W., N.K. and S.C. carried out the work. G.C. consented the patients. Y.D. recorded an ICM isolation movie.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Video 1
Time-lapse movie showing development of human 2PN embryo into fully expanded blastocyst. (MOV 7985 kb)
Supplementary Video 2
Micromanipulator set-up. (MOV 8376 kb)
Supplementary Video 3
Zona Pellucida (ZP) drilling with a stream of acid Tyrode's solution. ICM, inner cell mass. Asterisk (*), thinning ZP and forming a hole through which ICM can be aspirated. (MOV 4178 kb)
Rights and permissions
About this article
Cite this article
Stephenson, E., Jacquet, L., Miere, C. et al. Derivation and propagation of human embryonic stem cell lines from frozen embryos in an animal product–free environment. Nat Protoc 7, 1366–1381 (2012). https://doi.org/10.1038/nprot.2012.080
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2012.080
This article is cited by
-
Comparison of human isogeneic Wharton’s jelly MSCs and iPSC-derived MSCs reveals differentiation-dependent metabolic responses to IFNG stimulation
Cell Death & Disease (2019)
-
High quality clinical grade human embryonic stem cell lines derived from fresh discarded embryos
Stem Cell Research & Therapy (2017)
-
Ten years of iPSC: clinical potential and advances in vitro hematopoietic differentiation
Cell Biology and Toxicology (2017)
-
Long-term xeno-free culture of human pluripotent stem cells on hydrogels with optimal elasticity
Scientific Reports (2015)
-
Systematic optimization of human pluripotent stem cells media using Design of Experiments
Scientific Reports (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.