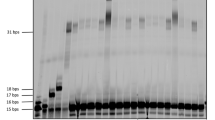
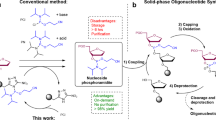
Abstract
Pseudo-complementary peptide nucleic acid (pcPNA) is a DNA analog in which modified DNA bases 2,6-diaminopurine (D) and 2-thiouracil (Us) 'decorate' a poly[N-(2-aminoethyl)glycine] backbone, together with guanine (G) and cytosine (C). One of the most significant characteristics of pcPNA is its ability to effect double-duplex invasion of predetermined DNA sites inducing various changes in the biological and the physicochemical properties of the DNA. This protocol describes solid-phase synthesis of pcPNA. The monomers for G and C are commercially available, but the monomers for D and Us need to be synthesized (or can be ordered to custom synthesis companies). Otherwise, the procedure is the same as that employed for Boc-strategy synthesis of conventional PNA. This protocol, if the synthesis of D and Us monomers is not factored in, takes approximately 7 d to complete.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Felsenfeld, G., Davies, D.R. & Rich, A. Formation of a 3-stranded polynucleotide molecule. J. Am. Chem. Soc. 79, 2023–2024 (1957).
Thuong, N.T. & Hélène, C. Sequence-specific recognition and modification of double-helical DNA by oligonucleotides. Angew. Chem. Int. Ed. Engl. 32, 666–690 (1993).
White, S., Szewczyk, J.W., Turner, J.M., Baird, E.E. & Dervan, P.B. Recognition of the four Watson–Crick base pairs in the DNA minor groove by synthetic ligands. Nature 391, 468–471 (1998).
Kielkopf, C.L. et al. A structural basis for recognition of A·T and T·A base pairs in the minor groove of B-DNA. Science 282, 111–115 (1998).
Lohse, J., Dahl, O. & Nielsen, P.E. Double duplex invasion by peptide nucleic acid: a general principle for sequence-specific targeting of double-stranded DNA. Proc. Natl. Acad. Sci. USA 96, 11804–11808 (1999).
Izvolsky, K.I., Demidov, V.V., Nielsen, P.E. & Frank-Kamenetskii, M.D. Sequence-specific protection of duplex DNA against restriction and methylation enzymes by pseudocomplementary PNAs. Biochemistry 39, 10908–10913 (2000).
Protozanova, E., Demidov, V.V., Nielsen, P.E. & Frank-Kamenetskii, M.D. Pseudocomplementary PNAs as selective modifiers of protein activity on duplex DNA: the case of type IIs restriction enzymes. Nucleic Acids Res. 31, 3929–3935 (2003).
Kuhn, H., Cherny, D.I., Demidov, V.V. & Frank-Kamenetskii, M.D. Inducing and modulating anisotropic DNA bends by pseudocomplementary peptide nucleic acids. Proc. Natl. Acad. Sci. USA 101, 7548–7553 (2004).
Haaima, G., Hansen, H.F., Christensen, L., Dahl, O. & Nielsen, P.E. Increased DNA binding and sequence discrimination of PNA oligomers containing 2,6-diaminopurine. Nucleic Acids Res. 25, 4639–4643 (1997).
Chollet, A. & Kawashima, E. DNA containing the base analogue 2-aminoadenine: preparation, use as hybridization probes and cleavage by restriction endonucleases. Nucleic Acids Res. 16, 305–317 (1988).
Kutyavin, I.V. et al. Oligonucleotides containing 2-aminoadenine and 2-thiothymine act as selectively binding complementary agents. Biochemistry 35, 11170–11176 (1996).
Nielsen, P.E., Egholm, M., Berg, R.H. & Buchardt, O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science 254, 1497–1500 (1991).
Egholm, M. et al. PNA hybridizes to complementary oligonucleotides obeying the Watson–Crick hydrogen-bonding rules. Nature 365, 566–568 (1993).
Komiyama, M., Aiba, Y., Yamamoto, Y. & Sumaoka, J. Artificial restriction DNA cutter for site-selective scission of double-stranded DNA with tunable scission-site and specificity. Nat. Protoc. 3, 655–662 (2008).
Watkins, B.E., Kiely, J.S. & Rapoport, H. Synthesis of oligodeoxyribonucleotides using N-(benzyloxycarbonyl)-blocked nucleosides. J. Am. Chem. Soc. 104, 5702–5708 (1982).
Christensen, L. et al. Solid-phase synthesis of peptide nucleic acids. J. Peptide Sci. 1, 175–183 (1995).
Acknowledgements
This work was partially supported by a Grant-in-Aid for Specially Promoted Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, and by Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists (for Y.A.). Support by the Global COE Program for Chemistry Innovation is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Komiyama, M., Aiba, Y., Ishizuka, T. et al. Solid-phase synthesis of pseudo-complementary peptide nucleic acids. Nat Protoc 3, 646–654 (2008). https://doi.org/10.1038/nprot.2008.6
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2008.6
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.