Abstract
Major depressive disorder (MDD) and nicotine dependence are highly comorbid, with studies showing that ~50% of individuals with MDD smoke. The link between these disorders persists even after the clinical symptoms of depression subside, as indicated by high levels of nicotine dependence among individuals with remitted depression (rMDD). Recent evidence indicates that individuals with rMDD show blunted responses to reward as measured by a probabilistic reward task (PRT), which assesses the ability to modify behavior as a function of reward history. Given nicotine’s ability to enhance reward responsiveness, individuals with rMDD might smoke to address this persistent reward deficit. However, it is unclear whether smokers with rMDD show enhanced reward responsiveness relative to rMDD individuals who do not smoke. To test this hypothesis, we evaluated reward responsiveness on the PRT in four groups (N=198): individuals with and without rMDD who were or were not nicotine dependent. As hypothesized, rMDD nonsmokers had lower reward responsiveness relative to both control nonsmokers and rMDD smokers; conversely, smokers with rMDD showed behavioral patterns comparable to those without a history of depression. Given nicotine’s ability to enhance reward sensitivity, it is possible that nicotine normalizes the otherwise blunted reward responsiveness in individuals with rMDD. Therapies aimed at enhancing this reward-based deficit may be beneficial in the treatment of both nicotine dependence and MDD.
Similar content being viewed by others
Introduction
The strong link between depression and cigarette smoking is well established. Individuals with major depressive disorder (MDD) are twice as likely to smoke compared with the general population (Diwan et al, 1998; Glassman et al, 1990); they experience greater withdrawal symptoms during abstinence, and are more likely to relapse to smoking following initial abstinence compared with smokers without depression (Glassman et al, 2001; Kinnunen et al, 1996). In addition, smoking cessation aids have been found to produce antidepressant effects (Salin-Pascual et al, 1995; Dwoskin et al, 2006; Hayford et al, 1999), suggesting that these disorders share underlying mechanisms. Of note, the link between MDD and nicotine dependence does not end when the clinical symptoms of MDD abate. Specifically, individuals with a lifetime history of depression continue to show heightened levels of nicotine dependence and difficulty in quitting even when depression remits (Trosclair and Dube, 2010; Niaura et al, 1999; Hitsman et al, 2013), indicating that the underlying pathophysiology common to both disorders persists during euthymic states.
Blunted reward function, or anhedonia, may be one point of overlap between these disorders. Anhedonic behavior is not only a symptom that can remain at subclinical levels during remitted depression (Pechtel et al, 2013) but is also a risk factor for smoking relapse (Leventhal et al, 2009) and cigarette craving (Cook et al, 2004). Whereas anhedonia is associated with a behavioral disruption in reward responsiveness (Pizzagalli et al, 2005), nicotine has the opposite effect and enhances reward sensitivity (Barr et al, 2008; Kenny and Markou, 2006). Furthermore, nicotine withdrawal results in diminished activity in brain reward systems (Kenny and Markou, 2006), and diminished reward responsiveness, which is particularly pronounced in individuals with remitted depression (rMDD; Pergadia et al, 2014). An interpretation of this latter finding is that nicotine withdrawal not only decreases reward reactivity but may also unmask depression-related reward dysfunction in individuals with rMDD. In addition, sated nicotine-dependent smokers with MDD show greater reward responsiveness relative to nonsmokers with MDD (Liverant et al, 2014), suggesting that nicotine may impact the reward sensitivity of individuals with MDD. Collectively, these findings suggest that, through nicotine’s action on reward function, smokers with rMDD may experience relatively higher levels of reward responsiveness compared with nonsmokers with rMDD. Although prior work has evaluated the interaction between rMDD and nicotine withdrawal, no research to date has compared reward responsiveness between smokers and nonsmokers with rMDD.
To address this gap, we measured reward responsiveness, a behavioral measure of hedonic function, in four groups of individuals using a 2 × 2 design: those with and without rMDD who were or were not nicotine dependent. Reward responsiveness was measured using a probabilistic reward task (PRT), which assesses the ability to modify behavior as a function of reward history (Pizzagalli et al, 2005). Given nicotine’s ability to enhance reward function, we hypothesized that current nicotine-dependent smokers with a history of depression would show greater reward responsiveness on the PRT relative to individuals with rMDD who do not use nicotine. This finding would support the notion that individuals with rMDD smoke to address a persistent deficiency in reward function, and suggests a mechanism through which depression history and nicotine dependence are linked and interact with each other.
Methods
Participants
A total of 198 women participated in this study. This population was subdivided into four groups: nicotine-dependent smokers without a history of MDD (control smokers, n=104), current nicotine-dependent smokers with a history of MDD (rMDD smokers, n=42), nonsmokers without a history of MDD (control nonsmokers, n=22), and nonsmokers with a history of MDD (rMDD nonsmokers, n=30). Group-specific demographics are outlined in Table 1. The data from current smokers were collected as part of a smoking-cessation clinical trial conducted at Massachusetts General Hospital (Evins et al, 2011), and all data from nonsmokers were collected at Harvard University and were part of a larger cohort discussed in the study by Pechtel et al (2013). All smokers were treatment-seeking for nicotine dependence and reported smoking a minimum of 10 cigarettes per day for the month before assessment, met DSM-IV criteria for nicotine dependence, and had smoked within the past 4 h relative to the time of testing (average time since last cigarette: 2.89±0.79 h). Smoking status was confirmed by expired carbon monoxide >10 ppm and saliva cotinine concentration >30 ng/ml. All participants in the nonsmoking sample had not had a cigarette for at least 30 days. Seven of the healthy nonsmokers reported having tried a cigarette at least once, as did 16 of the nonsmokers with rMDD. All participants were assessed for the presence of current DSM-IV diagnoses using the Structured Clinical Interview for DSM-IV (SCID) (First et al, 2002). Participants were excluded for having major depressive disorder within the prior 6 months, alcohol use disorder within the prior 6 months, current illicit psychotropic drug use, or lifetime diagnosis of organic mental or psychotic disorder. All rMDD subjects met criteria for past MDD that was in remission at the time of enrollment. Institutional Review Boards at Massachusetts General Hospital and Harvard University approved the two studies.
Assessment of Nicotine Dependence and Depressive Symptoms
Within the smoking sample, nicotine dependence severity was assessed using the Fagerstrom test for nicotine dependence. Nonsmokers were assessed using the tobacco use subscale of the Youth Risk Behavior Scale (Kolbe et al, 1993) and a general habit questionnaire, which contains questions about smoking habits.
Depression severity was measured with the 17-item Hamilton Rating Scale for Depression (HAM-D; Hamilton, 1960) in the smoking cohort and the 21-item Beck Depression Inventory-II (BDI-II; Beck et al, 1996) in the nonsmoking cohort. To adjust for the fact that different depression measures were used in the two samples, the scores from these assessments were converted into Z-scores based on population norms for statistical analyses.
PRT
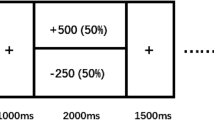
To probe reward responsiveness, all subjects completed a 25-min computer-based PRT (Pizzagalli et al, 2005). The PRT is a task rooted within signal detection theory that allows for an objective assessment of the propensity to modulate behavior as a function of prior reinforcements. The PRT consists of simple cartoon faces that are presented in the center of the monitor, each with two eyes and a straight horizontal line for a mouth. At the beginning of each trial, a fixation cross appears for 500 ms, followed by the face with no mouth. After a delay of 500 ms, either a ‘short mouth’ (11.5 mm) or a ‘long mouth’ (13 mm) is presented for 100 ms. Subjects are instructed to press a key to indicate whether the short or long mouth was presented. The PRT consists of three blocks of 100 trials, and a total of 40 correct trials in each block are followed by reward feedback with either a monetary (‘Correct!! You won 5 cents’) or social focus (‘Correct! Well done!’). Long mouths and short mouths are presented at equal frequencies; however, one of the mouth lengths (the ‘rich stimulus’) is rewarded three times more frequently than the other mouth length (the ‘lean stimulus’), although subjects are not informed about this contingency. Subjects were told that the aim of the task was to win as much money or get as much positive feedback as possible. At the conclusion of the task, those receiving monetary feedback were given the amount of money that they earned.
PRT Calculations and Quality Assessment
In line with prior studies using the PRT (eg, Pizzagalli et al, 2005, 2008), data were subjected to quality control analyses that involved excluding trials with reaction times of <150 ms or >1500 ms, as well as data from subjects who had accuracy scores below 55% (which would be indicative of chance level performance). Following exclusion of these outliers, signal detection analysis (Macmillan and Creelman, 1991) was used to calculate indices of response bias (the tendency to bias responding to the rich stimulus) and discriminability (the ability to accurately distinguish between the two mouth sizes) for each of the three blocks individually, as well as across the blocks. Accuracy was also calculated as the percentage of correct responses. Response bias and discriminability were calculated according to the following formula (note that in accordance to Hautus (1995), 0.5 was added to every cell of the detection matrix to allow for log transformation of cells with a value of zero):


Analyses
Univariate analyses of variance (ANOVA) were used to evaluate differences in demographics between the four groups (control smokers, rMDD smokers, control nonsmokers, and rMDD nonsmokers). To examine interactions between smoking status and depression history on PRT performance, mixed model repeated measures analysis of covariance (ANCOVA) with the within-subjects factor of block (block1, block2, and block3) and the between-subjects factors of depression history (control vs rMDD) and smoking status (smoker vs nonsmoker) was conducted with response bias and discriminability as dependent variables, and group differences on demographic variables as covariates. For the accuracy and reaction time variables of the PRT, an additional within-subjects factor of stimulus (rich vs lean) was included. To ensure that feedback type (monetary or social) had no impact on the data, this variable was included as a covariate. Significant ANOVA effects were followed up with tests of simple effects.
Results
Demographics
There were significant differences in age and years of education both between and within the smoking and nonsmoking groups (see Table 1). Smokers were significantly older than nonsmokers (46.0 vs 29.4 years, p<0.001), and had also completed fewer years of education (14.1 vs 16.2 years, p<0.001). Within the cohort of current smokers there was no significant difference between the control and the rMDD groups in the amount of time between their last cigarette and onset of the PRT task. Within the nonsmoking group, rMDD nonsmokers had completed fewer years of education compared with control nonsmokers (15.4 vs 17.0 years, p=0.005). Rates of anhedonic symptoms as reported on the SCID were also comparable between groups (see Supplementary Information). In light of these group differences, age and years of education were used as covariates in subsequent analyses. Within the smoking group, rMDD smokers started smoking regularly at younger age compared with control smokers (15.7 vs 17.0-years old, t(141)=1.97, p=0.050). To evaluate a possible association between age of smoking onset and reward responsiveness, a correlation analysis was conducted between these two measures within the smoking group.
Relationship Between Reward Responsiveness and Depression History
Response bias
The smoking status × depression history × block analysis revealed a significant smoking status × depression history interaction for response bias (F(1, 192)=4.69, p=0.03, ηp2=0.024) (Figure 1). Follow-up tests revealed that rMDD nonsmokers had lower response bias compared with control nonsmokers (p=0.045); however, rMDD smokers and control smokers did not differ (p=0.35). Moreover, relative to rMDD smokers, rMDD nonsmokers had a lower response bias (p=0.002), whereas response bias did not differ between control nonsmokers and control smokers (p=0.67). There was no main effect or interactions involving block (all ps>0.05). No relationship was found between age of smoking onset and reward responsiveness (p>0.05).
Significant smoking status by depression history interaction on overall response bias across blocks.
Discriminability
A main effect of depression history emerged from the ANOVA (F(1, 192)=4.09, p=0.04, ηp2=0.02). Across both smokers and nonsmokers, control subjects had higher discriminability compared with rMDD subjects. However, there was no smoking status × depression history interaction, indicating that the interaction effects observed for response bias were not simply driven by differences in the ability to differentiate between the two mouth sizes.
Accuracy
No block × stimulus × smoking status interaction emerged as significant.
Reaction time
No block × stimulus × smoking status interaction emerged as significant.
Control Analyses: Response Bias and Residual Depressive Symptoms
Although total average scores from the HAM-D did not differ between control and rMDD subjects in the smoking group, rMDD nonsmokers had significantly higher BDI-II scores compared with control nonsmokers (t(50)=2.54, p=0.01). Therefore, Z-scores for both measures were computed on the basis of population means and then entered into the above analyses as an additional covariate to control for potential effects of residual depressive symptoms on response bias. When residual depressive symptom severity was entered into the previous analyses as a covariate, the smoking status × depression history interaction (p=0.047) remained significant. This indicates that the interactive effect of smoking status and depression history on response bias was not influenced by higher residual depressive symptoms in rMDD individuals. Even after controlling for the smoking status × depressive symptom score, the interaction remained significant (F(1, 185)=3.99, p=0.047, ηp2=0.02).
Discussion
Evidence indicates that depression and nicotine dependence may share a common pathophysiology. Previously, we found that, among smokers, those in nicotine withdrawal with rMDD had relatively greater reward dysfunction (Pergadia et al, 2014). The fact that this prior study evaluated reward function following 24 h of abstinence made it difficult to determine whether rMDD-related reward dysfunction was due to pharmacological withdrawal or was an unmasking of preexisting reward dysfunction. The present results support the latter interpretation, as nonsmokers with rMDD had significantly blunted reward reactivity relative to rMDD smokers. In fact, smokers with rMDD had response bias levels comparable to those of never-depressed individuals, suggesting that smoking might normalize the blunted reward responsiveness commonly observed in those with a history of depression. In addition, no difference in response bias was found between the control groups regardless of smoking status, suggesting that chronic nicotine use does not raise response bias above those of nonsmoking controls.
It is possible that nicotine normalizes reward responsiveness in rMDD individuals through its effect on dopamine (DA). Specifically, nicotine increases the phasic firing of DA (Rice and Cragg, 2004; Zhang and Sulzer, 2004), which corresponds with a rapid burst of action potentials leading to a significant rise in DA at terminal projection sites (Grace and Bunney, 1984). Phasic DA firing is critical for reward-learning and motivated behavior (Schultz et al, 1997; Schultz, 2001), and disruptions in the DA reward circuit are associated with depressive symptoms, particularly anhedonia (Nestler and Carlezon, 2006; Pizzagalli, 2014). Critically, we previously showed that a pharmacological challenge hypothesized to blunt phasic DA lowered reward responsiveness on the PRT (Pizzagalli et al, 2008), suggesting a link between lowered phasic DA and disrupted reward reactivity during this task. In contrast, nicotine has reward-enhancing properties and increases the sensitivity to non-drug-related rewarding stimuli (Barr et al, 2008; Harrison et al, 2002; Kenny and Markou, 2006). Taken together, it is plausible that individuals with prior depression continue to have blunted phasic DA activity in response to rewarding stimuli during remission, leading to a disruption in reward reactivity. Should an individual with rMDD use nicotine, this deficit in phasic DA may be ameliorated, resulting in a relative normalization of reward responsivity during satiated states as shown in the present findings.
In addition to the interaction of nicotine dependence and depression history on response bias, we also found that rMDD smokers began smoking at a significantly earlier age compared with those without a history of depression (15.7 vs 17.0 years old). Analogously, rats that began self-administering nicotine during adolescence went on to self-administer more nicotine during adulthood (Levin et al, 2003), suggesting that nicotine use during this critical neurodevelopmental period may have long-term effects. It is difficult to disentangle reciprocal causal effects of these disorders, as a history of daily smoking increases the risk for developing MDD, whereas a history of MDD also increases the risk of progression to daily smoking (Breslau et al, 1998). It is possible that one disorder may not necessarily cause the other, but that nicotine dependence and MDD share a common mechanism that enhances the vulnerability for both (Markou et al, 1998). Given the earlier age of smoking onset for those with rMDD, it is possible that disruptions in this shared mechanism are evident even during adolescence.
Disrupted reward function appears to be one mechanism through which rMDD and nicotine dependence are linked. Blunted reward sensitivity, or anhedonia, is a risk factor for developing clinical depression (Loas, 1996, Meehl, 1975), but the present findings (along with preclinical research; Andreasen et al, 2011) suggest that nicotine may reverse anhedonic behavior. Whereas smokers with rMDD show a more profound reduction in reward responsiveness during abstinence (Pergadia et al, 2014), smokers with either current (Liverant et al, 2014) or remitted (current study) depression show a relative enhancement in their reward bias compared with nonsmoking counterparts. Collectively, these findings reveal a pattern of reduced reward sensitivity in those with current as well as past depression that is reversed by nicotine use in smokers. Thus, therapies that enhance reward function may be effective for both disorders and potentially necessary during smoking cessation attempts to prevent not only relapse to smoking but also reemergence of depressive symptoms.
When considering the present finding, the following limitations should be considered. First, this study was cross-sectional and thus firm causal inferences are not possible. Longitudinal studies evaluating reward responsiveness after sustained abstinence may further elucidate our findings. Given the persistence of reward dysfunction during remitted depression (Dichter et al, 2012; Pechtel et al, 2013) and the severity of nicotine withdrawal-induced reward dysfunction in rMDD (Pergadia et al, 2014), it is unlikely that the difference in response bias between rMDD smokers and rMDD nonsmokers was due to factors other than current nicotine use. Future work should include a more extensive examination of smoking history in the nonsmoker group to rule out any possible effect of past nicotine use on reward processing. Second, our sample included only female smokers; thus, further studies should examine whether findings will be replicated in male smokers. However, the focus on women is clinically relevant, as women show greater rates of depression (Kessler, 2003, Weissman et al, 1996; Nolen-Hoeksema, 1987) and lower rates of smoking cessation (Bjornson et al, 1995; Perkins, 2001; Perkins and Scott, 2008; Scharf and Shiffman, 2004). Third, the current analyses were conducted retrospectively using two data sets, leading to methodological differences between groups. However, such methodological differences as well as demographic differences such as age were controlled for in the analysis, indicating that these variations do not explain group differences. Finally, future studies evaluating reward function using larger cohorts and measures other than the PRT may expand our understanding of the impact of smoking on reward processing in those with a history of depression. Despite these limitations, the current work strengthens our knowledge of the link between MDD and nicotine dependence. Furthermore, it raises the possibility that nicotinic agents or agents that act up- or downstream of nicotinic receptors might improve the persistent reward system dysfunction observed in individuals with a lifetime history of MDD, and may therefore reduce the likelihood of relapse into smoking among rMDD patients.
Funding and Disclosure
This work was supported by National Institute of Health Grants U01DA019378 (MF), K01DA029645 (AJ), K24DA030443 (AEE) R10MH68376 (DP), R21MH078979 (DP), K23AA020064 (PP), and R01MH068376. Dr Evins has received research support in the form of funding and/or study supplies from GlaxoSmithKline, Pfizer, and Forum Pharmaceuticals. Over the past 2 years, Dr Pizzagalli has received honoraria/consulting fees from Otsuka America Pharmaceutical, Pfizer, and Servier for activities unrelated to this project. Maurizio Fava, M.D., Research Support: Abbot Laboratories; Alkermes, Inc.; American Cyanamid; Aspect Medical Systems; AstraZeneca; Avanir Pharmaceuticals; BioResearch; BrainCells Inc.; Bristol-Myers Squibb; CeNeRx BioPharma; Cephalon; Clintara, LLC; Covance; Covidien; Eli Lilly and Company;EnVivo Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Forest Pharmaceuticals, Inc.; Ganeden Biotech, Inc.; GlaxoSmithKline; Harvard Clinical Research Institute; Hoffman-LaRoche; Icon Clinical Research; i3 Innovus/Ingenix; Janssen R&D, LLC; Jed Foundation; Johnson & Johnson Pharmaceutical Research & Development; Lichtwer Pharma GmbH; Lorex Pharmaceuticals; Lundbeck Inc.; MedAvante; Methylation Sciences Inc; National Alliance for Research on Schizophrenia & Depression (NARSAD); National Center for Complementary and Alternative Medicine (NCCAM); National Institute of Drug Abuse (NIDA); National Institute of Mental Health (NIMH); Neuralstem, Inc.; Novartis AG; Organon Pharmaceuticals; PamLab, LLC.; Pfizer Inc.; Pharmacia-Upjohn; Pharmaceutical Research Associates., Inc.; Pharmavite LLC;PharmoRx Therapeutics; Photothera; Reckitt Benckiser; Roche Pharmaceuticals; RCT Logic, LLC (formerly Clinical Trials Solutions, LLC); Sanofi-Aventis US LLC; Shire; Solvay Pharmaceuticals, Inc.; Stanley Medical Research Institute (SMRI); Synthelabo; Wyeth-Ayerst LaboratoriesAdvisory/Consulting: Abbott Laboratories; Affectis Pharmaceuticals AG; Alkermes, Inc.; Amarin Pharma Inc.; Aspect Medical Systems; AstraZeneca; Auspex Pharmaceuticals; Bayer AG; Best Practice Project Management, Inc.; BioMarin Pharmaceuticals, Inc.; Biovail Corporation; BrainCells Inc; Bristol-Myers Squibb; CeNeRx BioPharma; Cephalon, Inc.; Cerecor; CNS Response, Inc.; Compellis Pharmaceuticals; Cypress Pharmaceutical, Inc.; DiagnoSearch Life Sciences (P) Ltd.; Dinippon Sumitomo Pharma Co. Inc.; Dov Pharmaceuticals, Inc.; Edgemont Pharmaceuticals, Inc.; Eisai Inc.; Eli Lilly and Company; EnVivo Pharmaceuticals, Inc.; ePharmaSolutions; EPIX Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Fabre-Kramer Pharmaceuticals, Inc.; Forest Pharmaceuticals, Inc.; GenOmind, LLC; GlaxoSmithKline; Grunenthal GmbH; i3 Innovus/Ingenis; Janssen Pharmaceutica; Jazz Pharmaceuticals, Inc.; Johnson & Johnson Pharmaceutical Research & Development, LLC; Knoll Pharmaceuticals Corp.; Labopharm Inc.; Lorex Pharmaceuticals; Lundbeck Inc.; MedAvante, Inc.; Merck & Co., Inc.; MSI Methylation Sciences, Inc.; Naurex, Inc.; Neuralstem, Inc.; Neuronetics, Inc.; NextWave Pharmaceuticals; Novartis AG;Nutrition 21; Orexigen Therapeutics, Inc.; Organon Pharmaceuticals; Otsuka Pharmaceuticals; Pamlab, LLC.; Pfizer Inc.; PharmaStar; Pharmavite® LLC.; PharmoRx Therapeutics; Precision Human Biolaboratory; Prexa Pharmaceuticals, Inc.; Puretech Ventures; PsychoGenics; Psylin Neurosciences, Inc.; RCT Logic, LLC (formerly Clinical Trials Solutions, LLC); Rexahn Pharmaceuticals, Inc.; Ridge Diagnostics, Inc.; Roche; Sanofi-Aventis US LLC.; Sepracor Inc.; Servier Laboratories; Schering-Plough Corporation; Solvay Pharmaceuticals, Inc.; Somaxon Pharmaceuticals, Inc.; Somerset Pharmaceuticals, Inc.; Sunovion Pharmaceuticals; Supernus Pharmaceuticals, Inc.; Synthelabo; Takeda Pharmaceutical Company Limited; Tal Medical, Inc.; Tetragenex Pharmaceuticals, Inc.; TransForm Pharmaceuticals, Inc.; Transcept Pharmaceuticals, Inc.; Vanda Pharmaceuticals, Inc. Speaking/Publishing: Adamed, Co; Advanced Meeting Partners; American Psychiatric Association; American Society of Clinical Psychopharmacology; AstraZeneca; Belvoir Media Group; Boehringer Ingelheim GmbH; Bristol-Myers Squibb; Cephalon, Inc.; CME Institute/Physicians Postgraduate Press, Inc.; Eli Lilly and Company; Forest Pharmaceuticals, Inc.; GlaxoSmithKline; Imedex, LLC; MGH Psychiatry Academy/Primedia; MGH Psychiatry Academy/Reed Elsevier; Novartis AG; Organon Pharmaceuticals; Pfizer Inc.; PharmaStar; United BioSource, Corp.; Wyeth-Ayerst Laboratories.Equity Holdings: Compellis; PsyBrain, Inc.Royalty/patent, other income: Patent for Sequential Parallel Comparison Design (SPCD), which are licensed by MGH to Pharmaceutical Product Development, LLC (PPD); and patent application for a combination of Ketamine plus Scopolamine in Major Depressive Disorder (MDD). Copyright for the MGH Cognitive & Physical Functioning Questionnaire (CPFQ), Sexual Functioning Inventory (SFI), Antidepressant Treatment Response Questionnaire (ATRQ), Discontinuation-Emergent Signs & Symptoms (DESS), and SAFER; Lippincott, Williams & Wilkins; Wolters Kluwer; World Scientific Publishing Co. Pte.Ltd. The remaining authors declare no conflict of interest.
References
American Psychiatric Association (1994) Diagnostic and Statistical Manual of Mental Disorders, 4th edn. American Psychiatric Press: Washington, DC.
Andreasen JT, Henningsen K, Bate S, Christiansen S, Wiborg O (2011). Nicotine reverses anhedonic-like response and cognitive impairment in the rat chronic mild stress model of depression: comparison with sertraline. J Psychopharmacol 25: 1134–1141.
Barr RS, Pizzagalli DA, Culhane MA, Goff DC, Evins AE (2008). A single dose of nicotine enhances reward responsiveness in nonsmokers: implications for development of dependence. Biol Psychiatry 63: 1061–1065.
Beck AT, Steer RA, Ball R, Ranieri W (1996). Comparison of beck depression inventories -IA and II in psychiatric outpatients. J Pers Assess 67: 588–597.
Bjornson W, Rand C, Connett JE, Lindgren P, Nides M, Pope F et al (1995). Gender differences in smoking cessation after 3 years in the lung health study. Am J Public Health 85: 223–230.
Breslau N, Peterson EL, Schultz LR, Chilcoat HD, Andreski P (1998). Major depression and stages of smoking. Arch Gen Psychiatry 55: 161–166.
Cook JW, Spring B, McChargue D, Hedeker D (2004). Hedonic capacity, cigarette craving, and diminished positive mood. Nicotine Tob Res 6: 39–47.
Dichter GS, Kozink RV, McClernon FJ, Smoski MJ (2012). Remitted major depression is characterized by reward network hyperactivation during reward anticipation and hypoactivation during reward outcomes. J Affect Disord 136: 1126–1134.
Diwan A, Castine M, Pomerleau CS, Meador-Woodruff JH, Dalack GW (1998). Differential prevalence of cigarette smoking in patients with schizophrenic vs mood disorders. Schizophr Res 33: 113–118.
Dwoskin LP, Rauhut AS, King-Pospisil KA, Bardo MT (2006). Review of the pharmacology and clinical profile of bupropion, an antidepressant and tobacco use cessation agent. CNS Drug Rev 12: 178–207.
Evins AE, Pachas G, Mischoulon D, Urbanoski K, Carlini S, Sousa J et al (2011). A double-blind, placebo-controlled trial of the NMDA glycine site antagonist, GW468816, for prevention of relapse to smoking in females. J Clin Psychopharmacol 31: 587–602.
First MB, Spitzer RL, Gibbon M, Williams JBW Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) Biometrics Research, New York State Psychiatric Institute: New York; (2002).
Glassman AH, Covey LS, Stetner F, Rivelli S (2001). Smoking cessation and the course of major depression: a follow-up study. Lancet 357: 1929–1932.
Glassman AH, Helxer JE, Covey LS, Cottler LB, Stetner F, Tipp JE et al (1990). Smoking, smoking cessation, and major depression. JAMA 264: 1546–1549.
Grace AA, Bunney BS (1984). The control of firing pattern in nigral dopamine nurons: burst firing. J Neurosci 4: 2877–2890.
Hamilton M (1960). A rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56–62.
Harrison AA, Gasparini F, Markou A (2002). Nicotine potentiation of brain stimulation reward reversed by DH beta E and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology 150: 56–66.
Hautus MJ (1995). Corrections for extreme proportions and their biasing effects on estimated values of d’. Behav Res Methods 27: 46–51.
Hayford KE, Patten CA, Rummans TA, Schroeder DR, Offord KP, Croghan IT et al (1999). Efficacy of bupropion for smoking cessation in smokers with a former history of major depression or alcoholism. Br J Psychiatry 174: 173–178.
Hitsman B, Papandonatos GD, McChargue DE, DeMott A, Herrera MJ, Spring B et al (2013). Past major depression and smoking cessation outcome: a systematic review and meta-analysis update. Addiction 108: 294–306.
Kenny PJ, Markou A (2006). Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology 31: 1203–1211.
Kessler RC (2003). Epidemiology of women and depression. J Affect Disord 74: 5–13.
Kinnunen T, Doherty K, Militello FS, Garvey AJ (1996). Depression and smoking cessation: Characteristics of depressed smokers and effects of nicotine replacement. J Consult Clin Psychol 64: 791–798.
Kolbe LJ, Kann L, Collins JL (1993). Overview of the youth risk surveillance system. Public Health Reports 108: 2–10.
Leventhal AM, Waters AJ, Kahler CW, Ray LA, Sussman S (2009). Relations between anhedonia and smoking motivation. Nicotine Tob Res 11: 1047–1054.
Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS (2003). Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology 169: 141–149.
Liverant GI, Sloan DM, Pizzagalli DA, Harte CB, Kamholz BW, Rosebrock LE et al (2014). Associations among smoking, anhedonia, and reward learning in depression. Behav Ther 13: 2014.
Loas G (1996). Vulnerability to depression: a model centered on anhedonia. J Affect Discord 41: 39–53.
Macmillan NA, Creelman CD (1991) Detection Theory: A User’s Guide. Cambridge University Press: New York.
Markou A, Kosten TR, Koob GF (1998). Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology 18: 135–174.
Meehl PE (1975). Hedonic capacity: some conjectures. Bull Menninger Clin 39: 295–307.
Nestler EJ, Carlezon WA (2006). The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 59: 1151–1159.
Niaura R, Britt DM, Borrelli B, Shadel WG, Abrams DB, Goldstein MG (1999). History and symptoms of depression among smokers during a self-initiated quit attempt. Nicotine Tob Res 1: 251–257.
Nolen-Hoeksema S (1987). Sex differences in unipolar depression: evidence and theory. Psychol Bull 101: 259–282.
Pechtel P, Dutra SJ, Goetz EL, Pizzagalli DA (2013). Blunted reward responsiveness in remitted depression. J Psychiatr Res 47: 1864–1869.
Perkins KA (2001). Smoking cessation in women: special considerations. CNS Drugs 15: 391–411.
Perkins KA, Scott J (2008). Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine Tob Res 10: 1245–1251.
Pergadia ML, Der-Avakian A, D’Souza MS, Madden PAF, Heath AC, Shiffman S et al (2014). Association between nicotine withdrawal and reward responsiveness in humans and rats. JAMA Psychiatry 71: 1238–1245.
Pizzagalli DA (2014). Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol 10: 393–423.
Pizzagalli DA, Evins AE, Schetter EC, Frank MJ, Pajtas PE, Santesso DL et al (2008). Single dose of dopamine agonist impairs reinforcement learning in humans: behavioral evidence from a laboratory-based measure of reward responsiveness. Psychopharmacology 196: 221–232.
Pizzagalli DA, Jahn AL, O’Shea JP (2005). Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry 57: 319–327.
Rice ME, Cragg SJ (2004). Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci 7: 583–584.
Salin-Pascual RJ, de la Fuente JR, Galicia-Polo L, Drucker-Colin R (1995). Effects of transderman nicotine on mood and sleep in nonsmoking major depressed patients. Psychopharmacology 121: 476–479.
Scharf D, Shiffman S (2004). Are there gender differences in smoking cessation, whith and without bupropion? Pooled- and meta-analysis of clinical trials of Bupropion SR. Addiction 99: 1462–1469.
Schultz W (2001). Reward signaling by dopamine neurons. Neuroscientist 7: 293–302.
Schultz W, Dayan P, Montague PR (1997). A neural substrate of prediction and reward. Science 275: 1593–1599.
Trosclair A, Dube SR (2010). Smoking among adults reporting lifetime depression, anxiety, anxiety with depression, and major depressive episode, United States, 2005–2006. Addict Behav 35: 438–443.
Weissman MM, Bland RC, Canino GJ, Farav- elli C, Greenwald S, Hwu H-G et al (1996). Cross-national epidemiology of major depression and bipolar disorder. JAMA 276: 293–299.
Zhang H, Sulzer D (2004). Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci 7: 581–582.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
PowerPoint slides
Rights and permissions
About this article
Cite this article
Janes, A., Pedrelli, P., Whitton, A. et al. Reward Responsiveness Varies by Smoking Status in Women with a History of Major Depressive Disorder. Neuropsychopharmacol 40, 1940–1946 (2015). https://doi.org/10.1038/npp.2015.43
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2015.43
This article is cited by
-
Electrophysiological signatures of reward learning in the rodent touchscreen-based Probabilistic Reward Task
Neuropsychopharmacology (2023)
-
A cross-species assay demonstrates that reward responsiveness is enduringly impacted by adverse, unpredictable early-life experiences
Neuropsychopharmacology (2022)
-
Empirical validation of a touchscreen probabilistic reward task in rats
Translational Psychiatry (2020)
-
Nicotine normalizes cortico-striatal connectivity in non-smoking individuals with major depressive disorder
Neuropsychopharmacology (2018)
-
Risk of mortality during and after the 2011 Great East Japan Earthquake and Tsunami among older coastal residents
Scientific Reports (2017)