Abstract
Although the serotonin (5-hydroxytryptamine, 5-HT) neurotransmitter system has been implicated in modulating executive control processes such as attention, response inhibition, and behavioral flexibility, the contributions of particular serotonin receptors remain unclear. Here, using operant-based behavioral paradigms, we demonstrate that mice with genetically ablated 5-HT2C receptors (2CKO mice) display deficits in executive functions. 2CKO mice were impaired in the acquisition of a visuospatial attention task as assessed in the 5-choice serial reaction time task (5-CSRTT). In this task, 2CKO mice exhibited marked impairment of attentional processes, with normal response inhibition. We assessed dynamic changes in neurotransmitter levels within the nucleus accumbens (NAc) by in vivo microdialysis in task-performing animals. Extracellular dopamine concentrations were elevated in the NAc of 2CKO mice during task performance, indicating that 5-HT2C receptors impact dopamine homeostasis during a visuospatial attention task. These findings raise the possibility that disinhibition of mesolimbic dopamine pathways contributes to impaired attention and perturbed task performance in 2CKO mice. Additionally, in a spatial reversal learning task, 2CKO mice failed to improve their performance over a series of reversals, indicating that intact 5-HT2C receptor signaling is required to accurately respond to repeated changes in reward contingencies. In contrast to the 2CKO phenotype in the 5-CSRTT, wild-type mice treated with the 5-HT2C receptor antagonist SB242084 exhibited diminished response inhibition, suggesting differing effects of acute pharmacological blockade and constitutive loss of 5-HT2C receptor activity. Altogether, these findings provide insights into the serotonergic regulation of executive control processes and suggest that impaired 5-HT2C receptor signaling during development may predispose to executive function disorders.
Similar content being viewed by others
INTRODUCTION
Executive function refers to a collection of processes that exert control over cognitive capacities such as the ability to attend and focus (eg, divided and sustained attention), to suppress behavior (response inhibition), and to modify behavior according to changing demands (behavioral flexibility). Dysfunctions in executive control processes are core features in a range of neuropsychiatric and neurological disorders, including attention-deficit/hyperactivity disorder (ADHD), depression, obsessive–compulsive disorder, schizophrenia, and Tourette’s syndrome (Doyle, 2006; Floresco and Jentsch, 2010; Simpson et al, 2010). Clinical and preclinical data suggest that serotonin (5-hydroxytryptamine, 5-HT) system activity impacts executive control processes (Chamberlain et al, 2006; Cools et al, 2008; Fineberg et al, 2010). A number of preclinical reports indicate that reducing the availability of brain serotonin produces behavioral inflexibility and impulsivity, whereas the effects on attentional functioning are less clear (Harrison et al, 1997; Clarke et al, 2004; Winstanley et al, 2004; Floresco and Jentsch, 2010; Angoa-Perez et al, 2012).
It has been challenging, however, to elucidate the relevant signaling pathways and mechanisms, especially due to the heterogeneity of the serotonin system, which includes at least 14 distinct receptor subtypes. Among the implicated receptors, the 5-HT2C receptor subtype has been linked to neuropsychiatric disorders (Giorgetti and Tecott, 2004; Drago and Serretti, 2009) and has emerged as a possible contributor to the regulation of executive functions (Tsaltas and Boulougouris, 2011). Administration of the 5-HT2C receptor antagonist SB242084 increased premature responding in the 5-choice serial reaction time task (5-CSRTT), a paradigm used to evaluate the features of behavioral control, including visuospatial attention, response inhibition, and reaction latencies in animal models (Winstanley et al, 2004; Fletcher et al, 2007a; Robinson et al, 2008). In contrast, administration of another 5-HT2C receptor antagonist (SER-082) did not alter impulsive behavior in the same task (Koskinen et al, 2000; Talpos et al, 2006). A potential role for 5-HT2C receptors in attention is also supported by the analysis of genetically modified mice modeling the Prader–Willi syndrome (PWS). These animals express less active 5-HT2C receptor isoforms and display an attention deficit in the 5-CSRTT (Doe et al, 2009; Relkovic et al, 2010). In addition, 5-HT2C receptors have been implicated in behavioral flexibility, which is commonly examined by administering reversal learning tasks that assess the ability of animals to shift responding from a previously learned to new stimulus-reward contingencies (Boulougouris et al, 2008; Boulougouris and Robbins, 2010).
The 5-HT2C receptor has been revealed to mediate the inhibitory effects of serotonin on both mesolimbic and nigrostriatal dopaminergic pathways (Alex and Pehek, 2007; Di Giovanni et al, 2008). Pharmacological and genetic inactivation of 5-HT2C receptors causes electrophysiological, neurochemical, and behavioral phenotypes consistent with disinhibition of these pathways (Gobert et al, 2000; Hutson et al, 2000; Rocha et al, 2002; Abdallah et al, 2009). Numerous studies in humans, non-human primates, and rodents have established the existence of an inverted U-shaped relationship between executive function and dopaminergic activity, with either too much or too little activity resulting in performance deficits (Zahrt et al, 1997; Granon et al, 2000; Clatworthy et al, 2009). This raises the possibility that 5-HT2C receptors could impact executive function by modulating dopamine neurotransmission.
The interpretation of drug studies of 5-HT2C receptor’s effects on behavior is complicated by potential limitations in receptor subtype selectivity and duration of action of pharmacological agents. In addition, the 5-HT2C receptor has a diverse spectrum of pharmacological properties, including constitutive (ligand-independent) activity, pleiotropic intracellular signaling effects, and agonist-directed trafficking of receptors (Werry et al, 2008). Therefore, it is not surprising that different pharmacological agents elicit varying physiological and behavioral effects (Navailles and De Deurwaerdère, 2011), as reported for the two 5-HT2C receptor antagonists whose effects were investigated in the 5-CSRTT. To complement such pharmacological studies, we assessed mice with genetically inactivated 5-HT2C receptors (2CKO mice; Tecott et al, 1995) for their performance in the 5-CSRTT and in a serial spatial discrimination and reversal learning (SD/SR) task. 2CKO mice have been validated to be devoid of functional 5-HT2C receptors and they have been extensively characterized in diverse assays of behavior and physiology (Tecott et al, 1995; Lopez-Gimenez et al, 2002; Bonasera, 2011). This approach allowed us to examine the impact of 5-HT2C receptor inactivation on task acquisition, as pharmacological manipulations are typically conducted after subjects have been successfully trained on a given task (eg, after reaching stable baseline performance on the 5-CSRTT). Furthermore, in light of evidence that central dopaminergic pathways impact executive control processes and that 5-HT2C receptors modulate these pathways, we examined the effects of 5-HT2C receptor loss on dopamine dynamics using in vivo microdialysis during performance in the 5-CSRTT.
MATERIALS AND METHODS
Animals
Adult male mice hemizygous for a null mutation of the X-linked 5-HT2C receptor gene (2CKO) and wild-type (WT) littermates congenic on a C57BL/6J background were used in this study (Tecott et al, 1995). The mice were housed in a controlled environment on a 12-h light/dark cycle (lights on at 0700 hours). Behavioral testing was carried out between 1100 and 1700 hours during the animals’ light phase. During the experimental period, mice were placed on a 22-h water deprivation schedule with standard laboratory chow available ad libitum, except during testing sessions. This procedure maintains mice at ∼90% of their free-feeding/drinking body weight and has been shown to maximize task motivation and performance in mice (Humby et al, 2005). All experiments were performed in accordance with the guidelines of the National Institutes of Health Guide for Care and Use of Laboratory Animals and the University of California, San Francisco Institutional Animal Care and Use Committee.
Behavioral Apparatus
Training and testing were conducted in four mouse operant conditioning boxes located within light- and sound-attenuated chambers (Med Associates, St Albans, VT, USA). All boxes contained a curved rear wall equipped with five holes, each with a photocell beam located at the entrance to detect nose-poke responses. A 3-W stimulus light was located at the rear of each hole. The opposite wall was equipped with a magazine connected to a dipper for automatic delivery of a liquid reward (10% solution of condensed milk, Nestle, USA). An infrared photocell beam crossing the entrance of the food magazine recorded head entries into the magazine. Above the food magazine, the house light was situated, which was normally turned off during testing. The apparatus was controlled by a Smart Ctrl Package (8IN/16OUT) by MedPC-IV software for Windows, running a modified mouse version of the MED State Notation SOF-700RA-8A for the 5-CSRTT (Med Associates), or an in-house programmed serial SD/SR task version.
Behavioral Procedures
General guidance for habituation and training of rodents on operant nose-poke paradigms can be found in recent protocol articles (Humby et al, 2005; Bari et al, 2008). Detailed methods used in this study are provided in the Supplementary Material.
5-CSRTT training
After an animal was successfully trained to respond to a stimulus light in the center hole to trigger reward release (one-choice task), the test chamber was configured for the 5-CSRTT so that all five nose-poke holes were accessible. The animal was then required over a large number of consecutive trials (a training session lasted for 100 trials or 25 min, whichever came first) to detect a brief visual stimulus that was pseudorandomly presented in one of the five horizontally arrayed holes. Only a nose-poke response into the correct spatial location within a short predetermined period (limited hold=LH) allowed reward access. Responses made prior to the presentation of the stimulus (during the inter-trial interval=ITI) were considered as premature responses reflecting a type of motor impulsivity, defined as ‘the inability to withhold from making a response’. Premature responding, responding into a hole different from the one that had been illuminated (incorrect response), and errors of omission (no response within the LH), each resulted in a 5-s time-out period. To proceed to the next training stage, a mouse had to meet the performance criterion over two consecutive sessions, until the final baseline criterion with stimulus duration (SD=1 s) was reached (see Supplementary Table S1).
The main performance measures were the total number of trials, accuracy (ratio of correct responses/correct+incorrect responses), omissions (ratio of omission errors/correct+incorrect responses+omissions), premature responses (ratio of premature responses/the total number of trials), correct response latency (elapsed time from stimulus onset till a correct response was made), and reward latency (elapsed time from a correct response till the collection of the reward). In addition, perseverative responses (additional responses in any nose-poke hole after a correct response was already made) were recorded, although not punished with a time-out. For full details and analysis methods, see Supplementary Material.
Serial spatial SD/SR task
The serial spatial SD/SR task used in this study was adapted from an instrumental two-lever task previously described in rats (Boulougouris et al, 2008). Operant conditioning boxes were configured to accommodate a two-hole discrimination task, where nose-poking was only possible in two spatial locations. In this procedure, a stimulus light was presented in two locations at the same time (LH=60 s) and the animals had to learn that nose-poking in either the left or the right hole was associated with reward delivery, whereas a response at the other location triggered a 5-s time-out period. After a response, both stimulus lights extinguished and a new trial was initiated after a 2-s ITI. The position of the reinforced nose-poke hole was kept constant for each mouse, but was counterbalanced between subjects and genotypes. Each session lasted for 20 min or consisted of a maximum of 80 trials, with one session per day. To complete the initial discrimination phase, mice were required to achieve a criterion of eight consecutive correct responses within a session, after which the run automatically stopped.
For reversal learning sessions, animals were again required to recall the initial learned discrimination by achieving the same criterion of eight consecutive correct responses. After the animals completed the retention phase, they were subjected to the reversal (within-session reversal). From this point on, the new reinforced location stayed the same until the subject successfully acquired the reversal criterion of eight consecutive correct responses within a session. A total of three reversals were given.
The number of trials to the criterion, the number of incorrect responses (errors) to the criterion, and the percentage of omitted trials (no response within the LH) were determined. Furthermore, errors made during the reversal stages were broken down into two types: perseverative errors (six or more consecutive errors within a session) and learning errors (all other errors) (Boulougouris and Robbins, 2010).
Drugs
Following establishment of stable 5-CSRTT baseline performance, the selective 5-HT2C receptor antagonist SB242084 (Tocris Bioscience, Ellisville, MO, USA) was administered using standard (5 s ITI) and increased ITI (7 s) conditions. Each manipulation was performed after two consecutive days of stable baseline performance. SB242084 was dissolved in 0.9% NaCl containing 8% hydroxypropyl-β-cyclodextrin and 25 mM citric acid, and the pH was adjusted using 0.1 M NaOH. The dosing volumes were 3 ml/kg, administered intraperitoneally 30 min before testing. Drug administrations were counterbalanced for genotype according to a Latin-square crossover drug design.
In vivo Microdialysis
Following drug administration studies, animals were prepared for the in vivo microdialysis experiment. I-shaped dialysis probes (NO-NM-PAN 6/1, polyacrylonitrile membrane, 1 mm exposed; Brainlink, Groningen, The Netherlands) were stereotactically implanted into the nucleus accumbens (NAc) under anesthesia (2% isofluorane and O2; analgesia: marcaine (0.5% topical) and carprofen (5 mg/kg s.c. pre-operation)), according to the following coordinates relative to bregma: AP +1.4 mm, L −0.9 mm, DV −4.5 mm. The probes were permanently fixed to the skull using stainless steel screws and dental cement. After surgery, the animals were housed individually and after a recovery period of 48 h they resumed testing on the 5-CSRTT. After 2–3 days of added training, a final performance day with sampling collection was initiated: The probes were first perfused with artificial cerebrospinal fluid containing 147.0 mM NaCl, 3.0 mM KCl, 1.2 mM MgCl2, and 1.2 mM CaCl2, at a flow rate of 1.5 μl/min (Harvard Apparatus Pump, South Natick, MA, USA). After a 2-h stabilization period, microdialysis samples were collected at 10-min intervals in HPLC vials containing 15 μl of 0.02 M formic acid for analysis. After collection of three basal samples in their home cage, animals were transferred to the operant conditioning chambers and the 5-CSRTT was initiated. After a 30-min 5-CSRTT session (corresponding to three task samples), the animals were returned to their home cage and monitored for additional 40 min (collection of four post-task samples). At the end of the sampling period, animals were killed. Perfusate analysis was carried out by liquid chromatography combined with tandem mass spectrometry (see Supplementary Material for detailed information). Within the same sample, dopamine, norepinephrine, and serotonin levels were analyzed.
RESULTS
A first cohort of 12-weeks-old WT (N=22) and 2CKO (N=18) mice were subjected to water deprivation and trained on the 5-CSRTT over the course of 6 months. A second cohort of 25-weeks-old WT (N=12) and 2CKO (N=14) mice were trained on the serial SD/SR task. Four mutant mice in the 5-CSRTT cohort and two mice in the SD/SR cohort died before finishing the experiments (no spontaneous death occurred in WT mice), and data from these animals were excluded from the analysis.
5-CSRTT
Acquisition to baseline
To study the consequences of constitutive 5-HT2C receptor deficiency on independent measures of behavioral control, including visuospatial attention and response inhibition, we first investigated the performance of 2CKO mice in the 5-CSRTT. Initial habituation training and shaping sessions in the operant conditioning chambers revealed no significant genotype differences (data not shown). In addition, both groups accomplished the one-choice task in less than 10 sessions on average (WT: 9.1±1.1, 2CKO: 9.8±1.1; Mann–Whitney U test, p>0.28, Figure 1a) and were similar in all parameters measured. Overall, these results indicate that the required cognitive, sensory, and motor abilities to perform the task were not impaired by global inactivation of 5-HT2C receptors.
5-CSRTT acquisition performance. (a) The number of sessions required to reach the criterion on the one-choice version of the task did not differ between genotypes (WT, N=22; 2CKO, N=14). (b) Cumulative percentage of mice in each group acquiring baseline criterion. (c) The mean number of sessions required to reach stable baseline performance was significantly higher in the 2CKO group. *p⩽0.05. Data shown represent mean±SEM (WT, N=20; 2CKO, N=8).
In contrast, a significantly higher percentage of 2CKO mice failed to acquire the baseline performance criterion (SD=1 s) on the 5-choice version of the task (WT mice: 9.1% (2/22), 2CKO mice: 32.9% (6/14); χ2(1, N=36)=5.64, p<0.05) (Figure 1b). Additionally, those 2CKO mice that reached the baseline criterion required significantly more sessions than WT mice (87.3±12.7 vs 60.7±5.6, Mann–Whitney U test, p<0.015, Figure 1c). Detailed analysis over the acquisition period using a linear mixed model with genotype and training stage as fixed effects revealed a significant effect of training stage on all parameters measured. In contrast, no genotype × training stage-specific interaction was detected, indicating that both genotypes adapted similarly to the task during training as the stimulus durations decreased. However, there was a significant increase in omission errors in 2CKO mice (main effect of genotype, F1,30.93=9.12, p<0.005, Figure 2a), with a strong trend towards a genotype × training stage interaction (F1,241.62=1.86, p<0.07). Overall, during the 5-CSRTT training, 2CKO mice failed to reach the omission criterion in 82% of all sessions, whereas WT mice missed the criterion in 60% of all sessions (F1,17=8.05, p<0.012, Figure 2b). No phenotypic differences were detected for the percentage of accuracy (main effect of genotype, F1,33.51=1.59, p>0.21), the total number of trials (F1,35.17=1.16, p>0.28), correct response latency (F1,29.15=0.09, p>0.76), as well as the percentage of premature responses (F1,36.78=0.72, p>0.40) (Supplementary Figure S1). In contrast, 2CKO mice demonstrated a significant decrease in the number of perseverative responses (F1,38.35=5.06, p<0.03) (Supplementary Figure S1) and in reward latency (main effect of genotype, F1,38.00=6.52, p<0.015, Figure 2c), suggesting intact motivation to complete the task. Thus, the impaired acquisition performance of 2CKO mice is primarily caused by their higher rate of omission errors that cannot be explained by insufficient motivation.
Detailed analysis of 5-CSRTT acquisition performance. (a) The percentage of omission errors was significantly enhanced in 2CKO mice. (b) In addition, the percentage of training sessions that did not meet the omission criterion was significantly higher in 2CKO mice. (c) A significant reduction in average reward latency, the main motivation index of the task, was detected in 2CKO mice. Only animals that reached the criterion on an individual training stage were used for calculations. Data shown represent mean±SEM.
Effect of SB242084
We did not observe abnormal premature responding in 2CKO mice during task acquisition or at baseline performance. In contrast, treatment with the selective 5-HT2C receptor antagonist SB242084 has previously been reported to increase premature responding in the 5-CSRTT (Winstanley et al, 2004; Fletcher et al, 2007a; Robinson et al, 2008). We therefore assessed whether SB242084 mediates its intrinsic effects on impulsivity indeed through 5-HT2C receptor signaling. In a first experiment (ITI=5 s), animals received either vehicle or SB242084 (0.25 and 0.5 mg/kg) 30 min before task onset. The results are summarized in Supplementary Table S2. SB242084 induced a significant increase in the percentage of premature responses (main effect of drug, F2,42=3.78, p<0.031, Figure 3a). Post hoc pairwise comparison revealed that, at the higher dose, the drug selectively increased impulsive responding only in WT mice (p<0.008), but not in the 2CKO group. In addition, a significant decrease in omission errors was noted (main effect of drug, F2,42=8.55, p<0.001). Subsequent analysis revealed that both doses significantly decreased omission errors in WT (0.25 mg/kg: p<0.001; 0.5 mg/kg: p<0.003), but not in 2CKO mice.
Effect of SB242084 on impulse control in the 5-CSRTT. (a) Under standard conditions (ITI=5 s), SB242084 at the higher dose (0.5 mg/kg) significantly increased premature responding in WT but not in 2CKO mice (WT, N=17; 2CKO, N=6). (b) By increasing the ITI duration to 7 s, thereby provoking more premature responding, SB242084 again augmented impulsive responding only in WT mice (WT, N=16; 2CKO, N=6). Data shown represent mean±SEM. *p⩽0.05, **p⩽0.01.
In a second experiment the effects of a 0.5-mg/kg dose of the drug were evaluated under a prolonged ITI condition, which typically initiates a higher anticipatory rate in rodents. Under this condition, a significant increase in premature responding was observed in WT, but not in 2CKO mice (main effect of drug, F1,20=4.45, p<0.048; main effect of genotype, F1,20=6.29, p<0.021; but no genotype × drug interaction, F1,20=2.93, p>0.10, Figure 3b; Supplementary Table S2). These findings suggest that the SB242084-induced increase in premature response behavior does reflect involvement of 5-HT2C receptors in the regulation of this indicator of impulsivity.
In vivo microdialysis
Next, we examined monoamine neurotransmitter dynamics during 5-CSRTT performance. We choose to target the ventral striatum, as (1) optimal function of this region is crucial for 5-CSRTT performance (Cole and Robbins, 1989, 2) it is a major target area of the mesolimbic dopamine pathway, and (3) pharmacological manipulation of 5-HT2C receptor signaling in the NAc impacts both 5-CSRTT behavior and dopamine activity (Robinson et al, 2008; Navailles and De Deurwaerdère, 2011). The absolute monoamine concentrations are provided in Supplementary Table S3. Basal neurotransmitter levels, recorded in the home cage prior to the transition to the operant conditioning chambers, revealed no differences in dopamine (F1,14=0.13, p>0.72) and norepinephrine (F1,14=2.59, p>0.13) levels between the two groups. However, basal serotonin concentrations were enhanced in the NAc of 2CKO mice (F1,14=11.98, p<0.004) (Supplementary Figure S2).
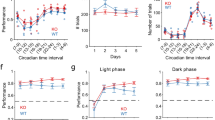
The effects of 5-CSRTT performance on dopamine and serotonin levels are shown in Figure 4. A two-way ANOVA (task phase × genotype) on the area under the curve values for dopamine revealed a significant effect of genotype (F1,14=5.03, p<0.042) and task (F1.61,22.5=6.25, p<0.01), as well as a genotype × task interaction (F1.61,22.5=3.62, p=0.05) (Figures 4a and c). Post hoc analysis indicated that dopamine efflux significantly increased during task performance only in 2CKO mice (p<0.002). In addition, extracellular dopamine concentrations remained elevated in 2CKO mice after the animals had returned to their home cage (p<0.04). Task performance also impacted the serotonin system, as we observed a modest reduction in extracellular serotonin compared to basal levels (F1.66,18.4=4.55, p<0.028) (Figures 4b and d). However, no genotype effect (F1,14=0.04, p>0.85) or genotype × task interaction (F1.66,18.4=0.50, p>0.58) was detected. No significant changes were detected in norepinephrine release. Overall, our results indicate that performance on the 5-CSRTT in the absence of 5-HT2C receptor signaling induces a hyperdopaminergic response in the ventral striatum.
Changes in neurotransmitter activity in the NAc during 5-CSRTT performance. Temporal patterns of (a) dopamine (DA) and (b) serotonin (5-HT) release are shown as percentage of baseline in 10-min sampling intervals. The time of 5-CSRTT task on-set is indicated with an arrow. (c, d) Bar graphs representing AUC (area under the curve) values for DA and 5-HT at baseline, during and after (post) task performance, showing (c) selective DA release upon task onset in 2CKO mice and (d) slightly reduced extracellular 5-HT levels independent of genotype. Data shown represent mean±SEM (WT, N=10; 2CKO, N=6). *p⩽0.05, **p⩽0.01.
Serial Spatial SD/SR Task
Because 5-HT2C receptor signaling has also been implicated in reversal learning (Boulougouris et al, 2008; Boulougouris and Robbins, 2010), we examined the performance of 2CKO and WT mice on a serial SD/SR task. As for the 5-CSRTT, initial habituation training and shaping sessions in the operant conditioning chambers (data not shown), as well as training on the one-choice task (sessions to criterion; WT: 10.5±0.6, 2CKO: 10.8±0.9; F1,23=0.04, p>0.84), did not reveal any significant differences between the two genotypes. Moreover, both groups readily acquired the performance criterion for the two-hole spatial discrimination challenge, as measured by the total number of trials (WT: 71.8±12.2, 2CKO: 84.0±18.4; F1,23=0.06, p>0.80) (Figure 5a) and the number of incorrect responses (WT: 32.6±5.9, 2CKO: 27.1±6.7; F1,23=0.06, p>0.81) (Supplementary Figure S3a). In addition, retention of the previously acquired discrimination and of subsequent reversals (three retention phases in total) were not significantly altered in 2CKO mice (total number of trials: main effect of retention, F1.65,36.21=0.35, p>0.66; genotype × retention, F1.65,36.21=1.30, p>0.28; main effect of genotype, F1,22=0.07, p>0.79; number of incorrect responses: main effect of retention, F2,44=1.14, p>0.33; genotype × drug interaction, F2,44=1.20, p>0.31; main effect of genotype, F1,22=0.001, p>0.97) (Figure 5a). On the contrary, when the reward contingencies were reversed, 2CKO mice required significantly more sessions to obtain criterion responding levels in all three stages of the SD/SR task (F1,22=11.29, p<0.003; Supplementary Figure S3b). Repeated-measures ANOVA across the three reversals revealed a significant impairment in reversal learning in 2CKO mice, with significantly more trials (main effect of genotype, F1,22=10.21, p<0.004) and incorrect responses (main effect of genotype, F1,22=10.75, p<0.003) to the criterion than WT controls (Figure 5). Post hoc analysis showed that 2CKO mice required significantly more trials and made more incorrect responses in reversal phase 2 (total number of trials, F1,23=7.48, p<0.012; number of incorrect responses, F1,23=5.71, p<0.026) and in reversal phase 3 (total number of trials, F1,23=9.43, p<0.006; number of incorrect responses, F1,23=12.34, p<0.002) than WT mice. Interestingly, similarly to the acquisition phase of the 5-CSRTT, 2CKO mice exhibited a significant increase in omission errors (main effect of genotype, F1,22=17.12, p<0.001). However, this phenotypic increase was seen during all stages of the SD/SR task and was therefore not directly related to the reversal deficit (Supplementary Figure S3c). Detailed analysis of the error types made during reversals revealed that 2CKO mice made more ‘perseverative errors’ (failure to inhibit responding to the previously rewarded stimulus; main effect of genotype, F1,22=12.92, p<0.002), as well as ‘learning errors’ (errors related to the acquisition and maintenance of a new stimulus-reward contingency; main effect of genotype, F1,22=7.75, p<0.011) (Supplementary Figure S4). Overall, these findings indicate that functional 5-HT2C receptors are required to accurately improve responding following imposition of repeated shifts in reward contingency.
Serial spatial SD/SR task performance. (a) The number of trials required to complete each stage of the serial SD/SR task. (b) The number of incorrect responses (errors) to the criterion across all three successive reversals, showing that 2CKO mice in contrast to WT mice did not improve their performance across reversals. SD, simple discrimination; SR, simple reversal; Ret, retention of a previously acquired stimulus–reward contingency. Data shown represent mean±SEM (WT, N=12; 2CKO, N=12). *p⩽0.05; **p⩽0.01.
DISCUSSION
In the current study, we report that genetic and pharmacological suppression of 5-HT2C receptor signaling perturbs the diverse aspects of executive function known to be dysregulated in several major psychiatric disorders. 2CKO mice exhibited impaired performance in the acquisition phase of the 5-CSRTT, in a manner indicative of a substantial deficit in attentional function. During performance of this task, 2CKO mice exhibited elevated extracellular dopamine levels in the NAc, a finding in accord with prior evidence that 5-HT2C receptors suppress ascending dopaminergic pathways (Rocha et al, 2002; Abdallah et al, 2009) and that perturbations of NAc dopamine signaling can impair attention (Cole and Robbins, 1989; Pezze et al, 2007). In addition to impaired acquisition in the 5-CSRTT, 2CKO mice also displayed impaired serial reversal learning. Interestingly, the phenotype of 2CKO mice in the 5-CSRTT was not mimicked by treatment with the 5-HT2C receptor antagonist SB242084, which enhanced premature responses in WT, but not in 2CKO mice. This discrepancy between the impact of constitutive loss of 5-HT2C receptors and that of pharmacological blockade raises the possibility that 5-HT2C receptor signaling is required for the proper development of neural pathways mediating normal executive function.
The most apparent alteration during acquisition training in the 5-CSRTT was the increased frequency of omitted trials in 2CKO mice. Such a result could reflect either diminished motivation or diminished attention (Spinelli et al, 2004; Yan et al, 2011). Several findings indicate that the elevated rate of omissions was not due to reduced motivation. First, animals were required to nose-poke into the food magazine to start a trial, so that new trial initiation was linked to reward seeking. Second, the increased omission rate in the mutant mice was not associated with a reduction in the total number of trials. And third, the collection latency of the reward, considered a measure of motivation in the 5-CSRTT, was in fact decreased in 2CKO mice. In light of these findings, it is likely that the increase in omission errors made by 2CKO mice reflects a gross deficit in attentional function. This deficit appears to generalize across tasks, as indicated by the increased omission errors exhibited by 2CKO mice in the SD/SR task.
In addition to a perturbation of attentional processes, behavioral flexibility was also impaired in 2CKO mice. We observed that these animals displayed normal initial discrimination learning and retention of an acquired stimulus-reward contingency in the SD/SR task, along with normal performance on the first shift in reward contingency (first reversal). However, 2CKO mice subsequently failed to progressively improve their performance following imposition of repeated shifts in reward contingency. The results observed during initial discrimination training and the first reversal differ from those in a recent study utilizing 2CKO mice (Nilsson et al, 2012). In this study, 2CKO mice displayed improved performance during initial discrimination training compared to WT littermates, with fewer correct responses to the criterion. In the same study, 2CKO mice exhibited improved performance during single reversal training. Although the factors underlying the discrepancies between these results and our findings are unclear, procedural differences may have played a role. In particular, differences existed in the time allowed for animals to make responses, which highly influences the rate of omission errors (12 s, vs 60 s limited hold times). It is also notable that in the Nilsson et al (2012) study the phenotypic difference may relate to the poor spatial discrimination performance of the WT littermates of 2CKO mice, which exhibited omission error rates substantially higher than those of 2CKO mice and vehicle-treated C57BL/6 mice. Indeed, when the omission errors were excluded from the analysis of the full reversal experiment, no longer was a significant difference between 2CKO and WT mice reported (comparable to our first reversal). The factors underlying the poor performance of the WT group in the Nilsson et al (2012) study are unclear.
Interestingly, the impairment in reversal learning was accompanied by increased perseverative responding (six or more consecutive errors within a session). In contrast, the perseveration index during 5-CSRTT acquisition (additional nose-poking after a correct response was already made) was diminished in 2CKO mice. It is possible that the differing impact of 5-HT2C receptor loss in the two assays relates to procedural differences. In the 5-CSRTT, perseverative responses had no programmed consequences (were not punished). In contrast, perseverative responses in the spatial reversal task were penalized by a 5-s time out. It is also noteworthy that there is precedence in the literature for experimental manipulations to differentially impact perseverative responding in a task-dependent manner. For example, the NMDA-receptor antagonist MK-801 was reported to selectively disrupt reversal learning in rats by increasing perseveration (van der Meulen et al, 2003), but also to decrease perseverative responding in the 5-CSRTT (Terry et al, 2012).
Much work has focused on the key roles played by ascending central dopamine pathways in the regulation of attention and other executive functions (Robbins and Arnsten, 2009; Floresco and Jentsch, 2010). It is therefore relevant that substantial evidence has established a prominent role for 5-HT2C receptors in mediating serotonergic suppression of both mesolimbic and nigrostriatal dopaminergic pathways (Alex and Pehek, 2007; Di Giovanni et al, 2008). In accord with this, 2CKO mice have been found to exhibit electrophysiological, neurochemical, and behavioral phenotypes consistent with disinhibition of these pathways (Rocha et al, 2002; Abdallah et al, 2009). In line with these data, we find that during performance of the 5-CSRTT, 2CKO mice exhibit an increase in dopamine efflux within the NAc. In light of this finding, it is notable that several studies have found activation of central dopamine pathways to impair attention. For example, systemic activation of dopamine D1 receptors (Bayer et al, 2000; Passetti et al, 2003) and amphetamine treatment (Cole and Robbins, 1987; Harrison et al, 1997; Dalley et al, 2005; Fletcher et al, 2007b, Pezze et al, 2007) produced significant increases in omitted trials in the 5-CSRTT. Moreover, an involvement of the mesolimbic dopamine system is suggested by the finding that amphetamine directly infused into the NAc increased omissions without affecting the accuracy of signal detection (Cole and Robbins, 1987). Altogether these studies raise the possibility that disinhibited activity of the mesolimbic dopamine system contributes to the attentional deficits in 2CKO mice; however, further studies are necessary in order to establish causality.
In addition to the marked impact of the 5-CSRTT procedure on extracellular NAc dopamine levels in 2CKO mice, the task produced small but significant reductions in 5-HT efflux in both 2CKO and WT mice. The suppression of 5-HT efflux during 5-CSRTT performance has not been previously reported, and neural mechanisms underlying this phenomenon are unclear. The possibility cannot be excluded that subtle phenotypic differences in serotonergic neurotransmission mediated by 5-HT receptors other than the 5-HT2C receptor may contribute to the observed behavioral phenotypes. Nevertheless, the observed decrease in 5-HT efflux during 5-CSRTT performance is less likely to underlie the phenotypic differences in task performance than the substantial enhancement of DA efflux, as there was no phenotypic difference in the extent to which 5-CSRTT performance diminished 5-HT efflux. It is also noteworthy that prior quantitative receptor autoradiography studies did not reveal significant alterations in the densities of other 5-HT receptor subtypes in any brain region examined in 2CKO mice (Lopez-Gimenez et al, 2002).
It remains to be established if other brain regions beside the NAc also show aberrant neurotransmitter signaling in 2CKO mice. In addition, alterations in NAc dopamine signaling do not per se indicate that the 5-HT2C receptor populations relevant for the observed executive function phenotypes are located in the ventral striatum. Indeed, in executive function tasks, a modulatory role of 5-HT2C receptors within the orbitofrontal cortex and dorsal medial striatum has been reported (Boulougouris and Robbins, 2010; Agnoli and Carli, 2012). Region-specific inactivation of 5-HT2C receptors in conditional mutant mice, together with complementary studies of locally injected 5-HT2C receptor ligands, could shed light on this issue.
Discrepancies exist between the phenotype of 2CKO mice and the effects of pharmacological blockade of 5-HT2C receptors in the 5-CSRTT. Antagonists such as SB242084 have not been reported to reliably impair measures of attention, but instead they increase premature responding in this task (Winstanley et al, 2004; Fletcher et al, 2007a; Robinson et al, 2008). Moreover, this drug has been shown to facilitate initial reversal learning, but did not impact adaptation to repeated shifts in reward contingency, in contrast to the impairment of serial reversal learning observed in 2CKO mice (Boulougouris et al, 2008). Here, we replicate prior observations that SB242084 increases premature responses in WT mice in the 5-CSRTT. In addition, we find that SB282084-induced increases in premature responding were absent in 2CKO mice, indicating that this behavioral effect of the drug is indeed 5-HT2C receptor-dependent.
We also note a discrepancy between the impact of 5-HT2C receptor gene inactivation and pharmacological blockade with regard to omission errors. Whereas 2CKO mice exhibited marked and persistent increases in omitted trials on both tasks, SB242084 treatment decreased omission errors under standard, but not under increased ITI conditions in the 5-CSRTT. Previously, SB242084-mediated reductions of omission errors had been reported in some, but not all studies (Winstanley et al, 2004; Fletcher et al, 2007a; Boulougouris et al, 2008; Robinson et al, 2008; Nilsson et al, 2012). In our 5-CSRTT study, SB242084-mediated reductions of omission errors in WT mice were accompanied by increases in numbers of trials performed and decreases in correct response latencies. These findings raise the possibility that SB242084 treatment enhanced motivational state in WT mice.
A number of factors can account for differences in the impact of htr2c gene inactivation and pharmacological blockade of 5-HT2C receptors on performance in the 5-CSRTT and SD/SR tasks. First, an important consideration in the interpretation of behavioral phenotypes in mice bearing constitutive null mutations is the possibility that they can reflect the effects of the mutation on brain development. In accord with this possibility, a large body of evidence indicates that genetic perturbations of components of the serotonin system are associated with impaired executive function (Chamberlain et al, 2006; Cools et al, 2008; Enge et al, 2011; Angoa-Perez et al, 2012). Second, in the pharmacological studies, drugs are typically administered after task acquisition had been performed in untreated animals. This contrasts with the situation in 2CKO mice, which lacked 5-HT2C receptor function throughout task acquisition. Finally, an additional consideration relates to the effects of SB242084 on 5-HT2C receptor signaling. Although this compound is considered to be an antagonist at 5-HT2C receptors, a study in cultured cells indicates that its actions may be more complicated (De Deurwaerdere et al, 2004). Whereas SB242084 was found to act as an inverse agonist (suppresses constitutive activity) with regard to the phospholipase A2 and Gαi pathways, it displayed mild agonist activity at the phospholipase C pathway (De Deurwaerdere et al, 2004). It is also notable that the effects of SB242084 treatment in freely behaving animals was found to differ from that of another 5-HT2C receptor compound (SB206553), which possesses inverse agonist activity for all three of the above-mentioned intracellular signaling pathways (De Deurwaerdere et al, 2004). Whereas SB206553 enhanced striatal dopamine efflux, SB242084 did not (Gobert et al, 2000; Hutson et al, 2000). However, it remains to be established how these drugs would affect dopamine release during performance of a visual spatial attention task.
In general, distinguishing the effects of stimulated vs constitutive actions of 5-HT2C receptors on tasks of executive function would be an interesting aim for future studies. Thereby, analysis of the behaviors elicited by SB206553 or shown by mutant mice with altered RNA editing patterns of 5-HT2C receptors would likely provide further insight into the role of 5-HT2C receptors in executive function. In this regard, it is notable that a 5-CSRTT phenotype has been recently reported in a mouse model with altered 5-HT2C receptor function. Mice engineered to recapitulate features of the PWS (PWS-IC+/− mice) were found to have increased 5-HT2C receptor editing, leading to the expression of less active receptor isoforms (Doe et al, 2009). Interestingly, similar to our findings, PWS-IC+/− mice required significantly more sessions to reach the baseline criterion in the 5-CSRTT. Moreover, attentional function was impaired, as indicated by increased omission errors and reduced accuracy at baseline (Relkovic et al, 2010). Although the genetic perturbation in PWS-IC+/− mice produces diverse effects beyond changes in 5-HT2C receptor expression, the similarities between the PWS mouse model and 2CKO mice further highlight a potential role of 5-HT2C receptors in the serotonergic modulation of visuospatial attention.
The current results indicate that the 5-HT2C receptor subtype modulates executive control processes, such as visuospatial attention and reversal learning. Evidence of enhanced NAc dopamine efflux in 2CKO mice during task performance raises the possibility that disinhibited mesolimbic dopamine system activity contributes to these behavioral deficits. A recent study in humans confirms a role for 5-HT2C receptors in regulating NAc dopamine release: an allelic variant of htr2c at the N-terminus of the receptor protein was associated with enhanced stress-induced striatal dopamine release (Mickey et al, 2012). The clinical relevance of our results is further highlighted by studies demonstrating that central serotonin and dopamine systems interact anomalously in ADHD (Oades, 2008). With regard to a possible involvement of 5-HT2C receptors in ADHD, two studies report changes in the allelic distribution of two polymorphisms in the promoter region of the 5-HT2C receptor gene in ADHD populations (Li et al, 2006; Xu et al, 2009), although not all studies have revealed an association (Bobb et al, 2005; Brookes et al, 2006). Although mutations resulting in the complete absence of 5-HT2C receptor expression have not been reported in humans, the potential relevance of 2CKO mice to ADHD is further suggested by the expression of several behavioral phenotypes relevant to this syndrome, including attentional impairment, reduced behavioral flexibility, physical hyperactivity, and obesity (Tecott et al, 1995; Itami and Uno, 2002; Nonogaki et al, 2003; Dempsey et al, 2011; Cortese and Vincenzi, 2012). Thus, it is possible that insights into how a more robust genetic perturbation (knockout) of 5-HT2C receptor function impacts executive function may facilitate attempts to understand the neural mechanisms through which common more subtle genetic alterations of 5-HT2C receptor function impact disease pathophysiology.
References
Abdallah L, Bonasera SJ, Hopf FW, O’Dell L, Giorgetti M, Jongsma M et al (2009). Impact of serotonin 2C receptor null mutation on physiology and behavior associated with nigrostriatal dopamine pathway function. J Neurosci 29: 8156–8165.
Agnoli L, Carli M (2012). Dorsal-striatal 5-HT(2)A and 5-HT(2)C receptors control impulsivity and perseverative responding in the 5-choice serial reaction time task. Psychopharmacology (Berl) 219: 633–645.
Alex KD, Pehek EA (2007). Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther 113: 296–320.
Angoa-Perez M, Kane MJ, Briggs DI, Sykes CE, Shah MM, Francescutti DM et al (2012). Genetic depletion of brain 5HT reveals a common molecular pathway mediating compulsivity and impulsivity. J Neurochem 121: 974–984.
Bari A, Dalley JW, Robbins TW (2008). The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat Protoc 3: 759–767.
Bayer LE, Brown A, Mactutus CF, Booze RM, Strupp BJ (2000). Prenatal cocaine exposure increases sensitivity to the attentional effects of the dopamine D1 agonist SKF81297. J Neurosci 20: 8902–8908.
Bobb AJ, Addington AM, Sidransky E, Gornick MC, Lerch JP, Greenstein DK et al (2005). Support for association between ADHD and two candidate genes: NET1 and DRD1. Am J Med Genet Part B 134B: 67–72.
Bonasera SJ (2011). Insights into 5-HT2C Receptor Function Gained from Transgenic Mouse Models. In: Di Giovanni G et al, (eds). The Receptors - 5-HT2C Receptors in the Pathophysiology of CNS Disease. Humana Press: New York. vol 22, pp 51–73.
Boulougouris V, Glennon JC, Robbins TW (2008). Dissociable effects of selective 5-HT2A and 5-HT2C receptor antagonists on serial spatial reversal learning in rats. Neuropsychopharmacology 33: 2007–2019.
Boulougouris V, Robbins TW (2010). Enhancement of spatial reversal learning by 5-HT2C receptor antagonism is neuroanatomically specific. J Neurosci 30: 930–938.
Brookes K, Xu X, Chen W, Zhou K, Neale B, Lowe N et al (2006). The analysis of 51 genes in DSM-IV combined type attention deficit hyperactivity disorder: association signals in DRD4, DAT1 and 16 other genes. Mol Psychiatry 11: 934–953.
Chamberlain SR, Muller U, Blackwell AD, Clark L, Robbins TW, Sahakian BJ (2006). Neurochemical modulation of response inhibition and probabilistic learning in humans. Science 311: 861–863.
Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC (2004). Cognitive inflexibility after prefrontal serotonin depletion. Science 304: 878–880.
Clatworthy PL, Lewis SJ, Brichard L, Hong YT, Izquierdo D, Clark L et al (2009). Dopamine release in dissociable striatal subregions predicts the different effects of oral methylphenidate on reversal learning and spatial working memory. J Neurosci 29: 4690–4696.
Cole BJ, Robbins TW (1987). Amphetamine impairs the discriminative performance of rats with dorsal noradrenergic bundle lesions on a 5-choice serial reaction time task: new evidence for central dopaminergic-noradrenergic interactions. Psychopharmacology (Berl) 91: 458–466.
Cole BJ, Robbins TW (1989). Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi on performance of a 5-choice serial reaction time task in rats: implications for theories of selective attention and arousal. Behav Brain Res 33: 165–179.
Cools R, Roberts AC, Robbins TW (2008). Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn Sci 12: 31–40.
Cortese S, Vincenzi B (2012). Obesity and ADHD: clinical and neurobiological implications. Curr Topics Behav Neurosci 9: 199–218.
Dalley JW, Theobald DE, Berry D, Milstein JA, Laane K, Everitt BJ et al (2005). Cognitive sequelae of intravenous amphetamine self-administration in rats: evidence for selective effects on attentional performance. Neuropsychopharmacology 30: 525–537.
De Deurwaerdere P, Navailles S, Berg KA, Clarke WP, Spampinato U (2004). Constitutive activity of the serotonin2C receptor inhibits in vivo dopamine release in the rat striatum and nucleus accumbens. J Neurosci 24: 3235–3241.
Dempsey A, Dyehouse J, Schafer J (2011). The relationship between executive function, AD/HD, overeating, and obesity. Western J Nurs Res 33: 609–629.
Di Giovanni G, Di Matteo V, Pierucci M, Esposito E (2008). Serotonin-dopamine interaction: electrophysiological evidence. Prog Brain Res 172: 45–71.
Doe CM, Relkovic D, Garfield AS, Dalley JW, Theobald DE, Humby T et al (2009). Loss of the imprinted snoRNA mbii-52 leads to increased 5htr2c pre-RNA editing and altered 5HT2CR-mediated behaviour. Hum Mol Genet 18: 2140–2148.
Doyle AE (2006). Executive functions in attention-deficit/hyperactivity disorder. J Clin Psychiatry 67 (Suppl 8): 21–26.
Drago A, Serretti A (2009). Focus on HTR2C: A possible suggestion for genetic studies of complex disorders. Am J Med Genet Part B 150B: 601–637.
Enge S, Fleischhauer M, Lesch KP, Strobel A (2011). On the role of serotonin and effort in voluntary attention: evidence of genetic variation in N1 modulation. Behav Brain Res 216: 122–128.
Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A et al (2010). Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology 35: 591–604.
Fletcher PJ, Tampakeras M, Sinyard J, Higgins GA (2007a). Opposing effects of 5-HT(2A) and 5-HT(2C) receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology (Berl) 195: 223–234.
Fletcher PJ, Tenn CC, Sinyard J, Rizos Z, Kapur S (2007b). A sensitizing regimen of amphetamine impairs visual attention in the 5-choice serial reaction time test: reversal by a D1 receptor agonist injected into the medial prefrontal cortex. Neuropsychopharmacology 32: 1122–1132.
Floresco SB, Jentsch JD (2010). Pharmacological enhancement of memory and executive functioning in laboratory animals. Neuropsychopharmacology 36: 227–250.
Giorgetti M, Tecott LH (2004). Contributions of 5-HT(2C) receptors to multiple actions of central serotonin systems. Eur J Pharmacol 488: 1–9.
Gobert A, Rivet JM, Lejeune F, Newman-Tancredi A, Adhumeau-Auclair A, Nicolas JP et al (2000). Serotonin(2C) receptors tonically suppress the activity of mesocortical dopaminergic and adrenergic, but not serotonergic, pathways: a combined dialysis and electrophysiological analysis in the rat. Synapse 36: 205–221.
Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW (2000). Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J Neurosci 20: 1208–1215.
Harrison AA, Everitt BJ, Robbins TW (1997). Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology (Berl) 133: 329–342.
Humby T, Wilkinson L, Dawson G (2005). Assaying aspects of attention and impulse control in mice using the 5-choice serial reaction time task. Curr Protoc Neurosci Chapter 8:Unit 8: 5H.
Hutson PH, Barton CL, Jay M, Blurton P, Burkamp F, Clarkson R et al (2000). Activation of mesolimbic dopamine function by phencyclidine is enhanced by 5-HT(2C/2B) receptor antagonists: neurochemical and behavioural studies. Neuropharmacology 39: 2318–2328.
Itami S, Uno H (2002). Orbitofrontal cortex dysfunction in attention-deficit hyperactivity disorder revealed by reversal and extinction tasks. Neuroreport 13: 2453–2457.
Koskinen T, Ruotsalainen S, Puumala T, Lappalainen R, Koivisto E, Mannisto PT et al (2000). Activation of 5-HT2A receptors impairs response control of rats in a five-choice serial reaction time task. Neuropharmacology 39: 471–481.
Li J, Wang Y, Zhou R, Zhang H, Yang L, Wang B et al (2006). Association between polymorphisms in serotonin 2C receptor gene and attention-deficit/hyperactivity disorder in Han Chinese subjects. Neurosci Lett 407: 107–111.
Lopez-Gimenez JF, Tecott LH, Palacios JM, Mengod G, Vilaro MT (2002). Serotonin 5-HT (2C) receptor knockout mice: autoradiographic analysis of multiple serotonin receptors. J Neurosci Res 67: 69–85.
Mickey BJ, Sanford BJ, Love TM, Shen PH, Hodgkinson CA, Stohler CS et al (2012). Striatal dopamine release and genetic variation of the serotonin 2C receptor in humans. J Neurosci 32: 9344–9350.
Navailles S, De Deurwaerdère P (2011). The Constitutive Activity of 5-HT2C Receptors as an Additional Modality of Interaction of the Serotonergic System. In: Di Giovanni G, et al (eds). The Receptors - 5-HT2C Receptors in the Pathophysiology of CNS Disease. Humana Press: New York, vol. 22 pp 187–213.
Nilsson SR, Ripley TL, Somerville EM, Clifton PG (2012). Reduced activity at the 5-HT(2C) receptor enhances reversal learning by decreasing the influence of previously non-rewarded associations. Psychopharmacology (Berl) 224: 241–254.
Nonogaki K, Abdallah L, Goulding EH, Bonasera SJ, Tecott LH (2003). Hyperactivity and reduced energy cost of physical activity in serotonin 5-HT(2C) receptor mutant mice. Diabetes 52: 315–320.
Oades RD (2008). Dopamine-serotonin interactions in attention-deficit hyperactivity disorder (ADHD). Prog Brain Res 172: 543–565.
Passetti F, Dalley JW, Robbins TW (2003). Double dissociation of serotonergic and dopaminergic mechanisms on attentional performance using a rodent five-choice reaction time task. Psychopharmacology (Berl) 165: 136–145.
Pezze MA, Dalley JW, Robbins TW (2007). Differential roles of dopamine D1 and D2 receptors in the nucleus accumbens in attentional performance on the five-choice serial reaction time task. Neuropsychopharmacology 32: 273–283.
Relkovic D, Doe CM, Humby T, Johnstone KA, Resnick JL, Holland AJ et al (2010). Behavioural and cognitive abnormalities in an imprinting centre deletion mouse model for Prader-Willi syndrome. Eur J Neurosci 31: 156–164.
Robbins TW, Arnsten AF (2009). The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci 32: 267–287.
Robinson ES, Dalley JW, Theobald DE, Glennon JC, Pezze MA, Murphy ER et al (2008). Opposing roles for 5-HT2A and 5-HT2C receptors in the nucleus accumbens on inhibitory response control in the 5-choice serial reaction time task. Neuropsychopharmacology 33: 2398–2406.
Rocha BA, Goulding EH, O'Dell LE, Mead AN, Coufal NG, Parsons LH et al (2002). Enhanced locomotor, reinforcing, and neurochemical effects of cocaine in serotonin 5-hydroxytryptamine 2C receptor mutant mice. J Neurosci 22: 10039–10045.
Simpson EH, Kellendonk C, Kandel E (2010). A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron 65: 585–596.
Spinelli S, Pennanen L, Dettling AC, Feldon J, Higgins GA, Pryce CR (2004). Performance of the marmoset monkey on computerized tasks of attention and working memory. Brain Res Cogn Brain Res 19: 123–137.
Talpos JC, Wilkinson LS, Robbins TW (2006). A comparison of multiple 5-HT receptors in two tasks measuring impulsivity. J Psychopharmacol 20: 47–58.
Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF et al (1995). Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature 374: 542–546.
Terry AV, Buccafusco JJ, Schade RF, Vandenhuerk L, Callahan PM, Beck WD et al (2012). The nicotine metabolite, cotinine, attenuates glutamate (NMDA) antagonist-related effects on the performance of the five choice serial reaction time task (5C-SRTT) in rats. Biochem Pharmacol 83: 941–951.
Tsaltas E, Boulougouris V (2011). The Role of Serotonin in Attentional Processes and Executive Functioning: Focus on 5-HT2C Receptors. In: Di Giovanni G, (eds). The Receptors - 5-HT2C Receptors in the Pathophysiology of CNS Disorders. Humana Press: New York, vol. 22 pp 445–459.
van der Meulen JA, Bilbija L, Joosten RN, de Bruin JP, Feenstra MG (2003). The NMDA-receptor antagonist MK-801 selectively disrupts reversal learning in rats. Neuroreport 14: 2225–2228.
Werry TD, Loiacono R, Sexton PM, Christopoulos A (2008). RNA editing of the serotonin 5HT2C receptor and its effects on cell signalling, pharmacology and brain function. Pharmacol Ther 119: 7–23.
Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW (2004). 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology (Berl) 176: 376–385.
Xu X, Brookes K, Sun B, Ilott N, Asherson P (2009). Investigation of the serotonin 2C receptor gene in attention deficit hyperactivity disorder in UK samples. BMC Res Notes 2: 71.
Yan TC, Dudley JA, Weir RK, Grabowska EM, Pena-Oliver Y, Ripley TL et al (2011). Performance deficits of NK1 receptor knockout mice in the 5-choice serial reaction-time task: effects of d-amphetamine, stress and time of day. PLoS One 6: e17586.
Zahrt J, Taylor JR, Mathew RG, Arnsten AF (1997). Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci 17: 8528–8535.
Acknowledgements
We thank Elaine Carlson, Ethelyn Layco, and Otis D Morgan III for technical assistance involved with these experiments. This work was funded by the SNSF-Swiss National Science Foundation—PBZH33-114661 (Pennanen), and Postdoctoral Research Fellowship from the American Foundation for Suicide Prevention (AFSP) (Pennanen).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Rights and permissions
About this article
Cite this article
Pennanen, L., van der Hart, M., Yu, L. et al. Impact of Serotonin (5-HT)2C Receptors On Executive Control Processes. Neuropsychopharmacol 38, 957–967 (2013). https://doi.org/10.1038/npp.2012.258
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2012.258
Keywords
This article is cited by
-
Association of Serotonin Receptors with Attention Deficit Hyperactivity Disorder: A Systematic Review and Meta-analysis
Current Medical Science (2018)
-
Dissociable Deficits of Executive Function Caused by Gestational Adversity are Linked to Specific Transcriptional Changes in the Prefrontal Cortex
Neuropsychopharmacology (2015)
-
The role of 5-HT2C receptors in touchscreen visual reversal learning in the rat: a cross-site study
Psychopharmacology (2015)