Abstract
Previous research has shown inconsistent findings regarding the relations between the functional Val158Met polymorphisms of the catechol-O-methyltransferase (COMT) gene and individual differences in personality traits. This study attempts to overcome some of the weaknesses of previous research, namely, small sample sizes, clinical samples, ethnic stratification, wide age ranges, neglecting sex differences, and single measures of personality traits. A large sample (n=556, 250 male, 306 female) of healthy Chinese college students (mean age=20.5±1 years) was given a battery of personality scales, including the temperament and character inventory-revised, the behavioral inhibition system and behavioral approach system scale, the Beck depression inventory, and the Beck anxiety inventory. Factor analysis of the affect-related personality traits revealed two factors that corresponded to positive (PEM) and negative emotionality (NEM). We found a consistent COMT-by-sex interaction effect on affect-related personality traits. Compared with males with Met/Met alleles, males with Val/Val alleles showed significantly higher scores on NEM, but lower scores on PEM. Females, however, showed an opposite but nonsignificant pattern. Our results supported the role of the COMT gene in personality traits for males and contributed to the growing literature on sex differences in gene–behavior connections.
Similar content being viewed by others
INTRODUCTION
Behavioral genetics research has long established that personality traits show a moderate to high level of heritability, suggesting a genetic basis for individual differences in those traits (eg, Bouchard 1994; Bouchard and Loehlin, 2001; Heiman et al, 2004; Lin et al, 2007). Recent advances in molecular genetics have allowed researchers to begin to investigate specific genes that are relevant to personality traits. The most commonly targeted genes are in the dopaminergic and serotonergic systems, including SLC6A4 (5HTTLPR), DRD4 (48-bp VNTR), and COMT (Val/Met; Munafo et al, 2003).
Catechol-O-methyltransferase (COMT) is an S-adenosylmethionine-dependent methyltransferase enzyme that methylates catechol substrates, notably catecholamines and catechol estrogens. This is a major pathway for dopamine inactivation. COMT has two isoforms: soluble (S-COMT) and membrane bound (MB-COMT). MB-COMT has approximately a 10-fold greater affinity for dopamine than S-COMT and predominates in the brain, reflecting its significant role in the prefrontal dopamine system. The COMT gene is located on chromosome 22q11, and has a well-known variation, a G → A substitution polymorphism (rs4680) that produces a valine-to-methionine (Val/Met) substitution at codons 108 and 158 of S-COMT and MB-COMT. This amino acid substitution influences the thermal stability and activity of COMT, such that the COMT activity in human prefrontal cortex is about 35–50% lower in Met homozygotes than in Val homozygotes (Chen et al, 2004). As a result, dopamine signaling is likely to be enhanced in Met carriers as compared with Val carriers, thus resulting in potential differences in prefrontal cognitive function (Savitz et al, 2006; Barnett et al, 2008; Goldman et al, 2009) and personality (Benjamin et al, 2000; Eley et al, 2003; Enoch et al, 2003; Tsai et al, 2004; Olsson et al, 2005; Reuter and Hennig, 2005; Stein et al, 2005; Kim et al, 2006; Reuter et al, 2006a; Tochigi et al, 2006; Golimbet et al, 2007; Hashimoto et al, 2007; Ishii et al, 2007; Lang et al, 2007; Light et al, 2007).
The COMT Val/Met effect on personality has been inconsistent across studies. One reason may be that personality traits have been measured with varying scales. Scales most often used to measure personality include the temperament and character inventory (TCI, or its older version tridimensional personality questionnaire; Cloninger et al, 1993), the five factor personality inventory (NEO; Costa and McCrae, 1992), and the behavior inhibition/approach system (BIS/BAS) questionnaire (Carver and White 1994). Recently, researchers have been able to group many affect-related traits into two categories. Traits such as harm avoidance (HA) in TCI, neuroticism in NEO, and BIS all belong to negative emotionality (NEM), whereas novelty seeking (NS) in TCI, extraversion in NEO, and BAS belong to positive emotionality (PEM; Reuter et al, 2006b). Associations between COMT Val/Met and PEM/NEM were quite mixed, with nine studies showing no significant associations (Strobel et al, 2003; Tochigi et al, 2006; Ishii et al, 2007; Light et al, 2007; Urata et al, 2007; Sheldrick et al, 2008; Calati et al, 2011; Kang et al, 2010; Salo et al, 2010), seven studies showing the Val allele associated with high PEM or low NEM (Enoch et al, 2003; Tsai et al, 2004; Reuter and Hennig, 2005; Stein et al, 2005; Hoth et al, 2006; Hashimoto et al, 2007; Wacker and Gatt, 2010), and three studies showing the opposite (Met allele associated with high PEM or low NEM; Kim et al, 2006; Golimbet et al, 2007; Demetrovics et al, 2010), and three studies reported opposite pattern for males and females (Eley et al, 2003; Reuter et al, 2006b; Lang et al, 2007).
Another reason for these inconsistent findings may be that, for association studies such as those mentioned above, statistical power is often an issue. A sample size of fewer than 200 individuals (including all genotypes), which is typical for previous studies, simply does not have sufficient statistical power to detect a true but modest association between a gene and personality (Long and Langley, 1999). Third, most studies have been carried out in countries such as the United States, where ethnic diversity is high, but ethnic stratification problems have often been ignored (Domschke et al, 2007). Fourth, health condition and the age of the subjects may have confounded some of the findings. For example, Rujescu et al (2003) found that the COMT gene's association with state and trait anger differed for healthy controls than for suicide attempters. Age is another potential confound because it is negatively correlated with traits such as NS (Ishii et al, 2007; Light et al, 2007) and HA (Hashimoto et al, 2007; Light et al, 2007). Biochemical studies have also reported that COMT activity level rises considerably in men between the third and fifth decade of life (Tunbridge et al, 2007).
Fifth, most studies used single measures of constructs instead of multiple measures that would have yielded more reliable data on personality traits. Although these measures may tap similar aspects of personality they are not exactly the same (Carver and White, 1994). This phenotype difference may have contributed to inconsistencies in results. Finally, and perhaps most importantly, sex may be an important confounding factor (Harrison and Tunbridge, 2008). Sex differences in COMT activity and in personality are well addressed in the literature (Carver and White, 1994; Cloninger et al, 2006; Darcan et al, 2008; Harrison and Tunbridge, 2008; Schmitt et al, 2008). In fact, several studies (Eley et al, 2003; Enoch et al, 2003; Reuter et al, 2006b; Lang et al, 2007) have reported sex-dependent COMT effects on personality, although others (Strobel et al, 2003; Reuter and Hennig 2005; Hoth et al, 2006; Hashimoto et al, 2007; Wacker and Gatt, 2010) reported no interactions between sex and the COMT gene.
To help clarify the role of the COMT gene in personality, studies need to overcome the above weaknesses. In this study, a large, homogeneous (in terms of ethnicity, age, education, and health status) sample was recruited: 556 healthy Chinese college students (250 males and 306 females), with a mean age of 20.5 years (SD=1.0). A wide range of personality traits was measured with multiple measures of related constructs. According to the PEM/NEM hypothesis (Reuter et al, 2006b), we selected novelty seeking and HA subscales of the TCI revised (TCI-R; Cloninger et al, 1993) and the behavioral inhibition system/behavioral approach system (BIS/BAS) scale (Carver and White, 1994). A warrior/worrier model was proposed regarding the function of the COMT Val/Met polymorphism (Stein et al, 2006). This model claims that the Val allele may be associated with advantages in processing aversive stimuli, thus Val carriers tend to exhibit decreased anxiety and better regulatory response to negative stimuli (warrior), whereas the Met allele may be associated with advantages in memory and attention tasks but also increased anxiety (worrier). Given the centrality of anxiety in this model, we also included in the current study the Beck anxiety inventory (Beck and Steer, 1990), and the Beck depression inventory second edition (Beck et al, 1996).
MATERIALS AND METHODS
Participants
In all, 556 healthy Chinese college students (250 males and 306 females, mean age=20.5 years, SD=1.0) participated in this study. Written informed consent was obtained from each participant. This experiment was approved by the IRB of the State Key Laboratory of Cognitive Neuroscience and Learning at Beijing Normal University, China.
Personality Measures
The following major personality measures were used in this study. The NS subscale of the TCI-R (Cloninger et al, 1993) measures individual differences in the extent to which a person is impulsive, quick tempered, and disorderly versus rigid, stoical, and orderly. The HA subscale of the TCI-R measures individual differences in the extent to which a person is anxious, pessimistic, and shy versus risk taking, optimistic, and outgoing. The BIS/BAS scale measures behavioral inhibition and approach systems (Carver and White, 1994). The BIS controls experience of anxiety in response to anxiety-relevant cues and is sensitive to signals of punishment, non-reward, and novelty; and inhibits behavior toward negative or painful outcomes. The BAS controls appetitive motivation and is sensitive to signals of reward and non-punishment. The Beck anxiety inventory and the Beck depression inventory (Beck and Steer, 1990; Beck et al, 1996) measure anxiety and depression, respectively.
All scales were translated from English to Chinese and back translated and verified through a bilingual group discussion. They were also pilot tested. The resulting Chinese versions all had high internal consistency. Furthermore, theoretically related constructs were correlated with one another, yielding construct/convergent validity (see Table 1). Specifically, BDI, BAI, HA, and BIS showed high correlations among them, which is consistent with the idea that they all measured NEM; NS and BAS were positively correlated but negatively correlated with the above scales, indicating that they measure PEM (see the Results section for confirming evidence from a factor analysis).
Genotyping
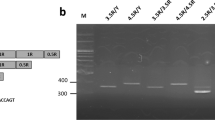
Subjects donated a blood sample through venipuncture. DNA was extracted and purified following the standard procedure. The genotypes of COMT val158met was determined in the following manner (see Qian et al, 2003). DNA fragments containing the Val158Met polymorphism in the COMT gene were amplified using PCR with primers (forward: 5′-TCGTGGACGCCGTGATTCAGG-3′ and reverse: 5′-ACAACGGGTCAGGCATGCA-3′). The PCR mixture consisted of 1 × Taq Buffer (MBI), 0.2 mM dNTP, 0.25 mM primers, 1 mM MgCl2, 1 U Taq polymorphism and 100 ng DNA. PCR was initiated at 95°C for 3 min, then at 94°C for 30 s, 68°C for 60 s, 72°C for 90 s for 35 cycles, then extended at 72°C for 7 min. The Val158Met variation was differentiated using NlaIII restriction fragment length polymorphism analysis on 4% agarose gel electrophoresis. Two common fragments (81 and 22 bp) and variable fragments of 114 bp (Val 158/wild type) and 96+18 bp (Met 158/mutant) were available. Genotypes were determined from Gel Doc 2000 (Bio-Rad, Hercules, CA). Because the short fragments (22 and 18 bp) diffused in the agarose gel, only three bands (114, 96, and 81 bp) were detectable. Ambiguous or unidentifible results were reamplified and rescored. A subset of the samples (10%) were randomly selected and tested twice for confirmation.
Among all 556 subjects, 310 were Val/Val, 210 were Val/Met, and 36 were Met/Met. The COMT genotype distribution in the overall sample was in the Hardy–Weinberg equilibrium (p=0.975). The frequencies of polymorphism for each sex are presented in Table 2. No distribution differences between males and females were found (χ2 (2)=2.298, p=0.317). Minor allele (A, code for met) frequency is 25%, which is comparable with the HapMap data (30% for Han Chinese in Beijing, China, and 30% for Chinese in Denver, CO).
Data Analysis
All data were analyzed using SPSS 15.0 for Windows. To alleviate the multiple comparisons problem and to combine diverse measurements to get a more reliable phenotype, exploratory factor analysis using the principal component extraction method followed by varimax rotation was carried out. Analysis of variance (ANOVA) was used to test the main effects and interaction effects of sex and the COMT Met/Val polymorphism on the latent phenotypes. Additional supplementary ANOVAs were conducted to explore which measures of the phenotypes made contributions to our results. When interaction effects were significant, simple effects tests and post hoc comparisons were conducted.
RESULTS
Exploratory factor analysis resulted in a two-factor solution, with Kaiser–Meyer–Olkin index=0.66, Bartlett's test χ2 (15)=961.132, p<0.001, together indicating that the solution is good. BDI, BAI, HA, and BIS had significant loadings on factor 1, which appeared to be consistent with the construct of NEM, accounting for 43% of the variance; and NS and BAS loaded on factor 2, which we labeled as PEM accounts for another 21% of the variance.
Table 3 shows the results of two-way ANOVA examining the effects of sex and the COMT gene Val/Met polymorphism on NEM and PEM. There were significant sex-by-gene interactions for both variables (see also Figure 1). Further one-way ANOVA revealed that these significant interactions mostly resulted from significant effects of the COMT gene on males’ traits, with an opposite but non-significant pattern for females. Compared with males with Val/Val alleles, males with Met/Met alleles showed significantly lower NEM and higher PEM. Females, in contrast, showed an opposite pattern for NEM (ie, Met/Met individuals had higher scores on NEM than Val/Val individuals), although it did not reach statistical significant in simple effects tests. To ascertain whether these sex-by-gene interactions were because of one or few specific measures or rather the general constructs, we conducted supplementary ANOVA for the six original measures. Results are shown in online Supplementary Table S1 and Figure S1. Briefly, five of the six measures showed significant interactions, indicating that the sex-by-gene interactions were not because of one or few specific measures, but rather the more general constructs we used. Specifically, further one-way ANOVA revealed that, compared with Met/Met males, Val/Val males showed significantly higher scores in anxiety, depression, behavioral inhibition, and HA, but lower scores in NS. Females showed a mostly opposite, but statistically nonsignificant, pattern (see Supplementary Table S1 and Figure S1).
Means of personality factors by genotype and sex.
Another way to appreciate the sex–COMT interactions is to examine sex differences for each genotype. Results showed that, for the Val/Val genotype, females consistently showed higher PEM than males, F(1,308)=10.48, p=0.001. For Val/Met and Met/Met (ie, Met present) genotypes, females showed higher NEM than males, F(1,255)=4.86, p=0.028.
DISCUSSION
Our main finding is that sex modulates the associations between the COMT gene's Val/Met polymorphism and personality traits. This finding adds to the growing literature on sex–COMT interaction effects on phenotypes ranging from psychiatric disorders (Harrison and Tunbridge, 2008) to personality traits such as sensation seeking (Lang et al, 2007) and behavioral inhibition system (Reuter et al, 2006b). Beyond the COMT gene and personality, of course, there is a growing literature of sex-dependent genetic effects on behavior, including performance on cognitive tests (O’Hara et al, 2006), nicotine dependence (Beuten et al, 2006), adolescent delinquency and thrill seeking (Dmitrieva et al, 2011), and ADHD (Qian et al, 2003).
The main division in personality traits included in our study is between traits representing NEM such as HA, BIS, BDI and BAI, and those (NS, BAS) representing PEM (Reuter et al, 2006b). We found that males and females generally had opposite patterns of association between the COMT gene and personality, resulting in significant sex differences among genotypes. For Val/Val carriers, sex differences occurred for PEM, and for Met carriers (both Val/Met and Met/Met), sex differences were evident for NEM.
According to the warrior/worrier model, Met carriers would have higher anxiety (or high NEM in the current study) than would Val/Val individuals. However, our data did not support this general prediction. Instead we found that the effect of the COMT gene differed by gender, which is consistent with Reuter et al (2006b) and Lang et al (2007). Specifically, for females, COMT's main effect on personality showed the predicted but nonsignificant trend, but for males, the effect of COMT was opposite of the prediction of the warrior/worrier model. Despite the findings of clear sex–COMT interactions across a number of studies, however, the patterns of specific interactions were not always consistent. Our results on the behavioral inhibition system were consistent with those by Reuter et al (2006b): Male Met carriers scored lower on BIS than male Val homozygotes. Our results further showed that this pattern was consistent across measures related to NEM such as BDI, BAI, BIS, and HA. Our results on sensation seeking, however, were inconsistent with the pattern found by Lang et al (2007) and Tsai et al (2004). Their studies found that female Val/Val carriers scored higher in sensation seeking than female Met/Met carriers, whereas we found no significant effect of the COMT gene for females (although the direction of differences were the same as the other two studies). Instead, we found that male Val/Met carriers were higher in sensation seeking than male Val/Val carriers. Lang et al (2007) did not find a significant COMT effect for males, and Tsai et al (2004) did not include males in their study.
These results have two significant implications. First, they point to the importance of including sex in analyses of gene–personality (perhaps broadly gene–behavior) associations. The role of sex in the relation between the COMT gene and personality has been discussed by Harrison and Tunbridge (2008). They suggested that estrogen may interact with COMT activity. It has been reported that estrogen inhibits COMT mRNA expression and reduces its activity (Xie et al, 1999; Jiang et al, 2003), consistent with an earlier study showing that women with high estrogen levels had lower COMT activity than other women (Briggs and Briggs, 1973). Moreover, there is evidence that COMT has an important role in metabolizing catechol estrogens and thereby lowering its levels (Creveling, 2003). Besides, Harrison and Tunbridge (2008) concluded that women appear to have elevated basal striatal dopamine levels, because, compared with men, women have greater presynaptic dopamine synthesis, greater dopamine transporter uptake, lower D2 receptor affinity, and lower amphetamine-stimulated dopamine release. These sex differences may interact with the COMT gene in their effects on personality.
Second, even when sex is considered in the analyses of gene–behavior associations, there are still inconsistencies in the findings across studies. One reason is that a single gene such as the COMT gene is only a part of the complex neural system (eg, the dopamine system). In the case of the dopamine system, other dopamine genes such as receptor genes (DRD1, DRD2, DRD3, DRD4, DRD5) and transporter genes (SLC6A3) and possibly other systems such as the serotonin system may all interact with the effects of the COMT gene. A comprehensive understanding of the genetic bases of personality needs to consider the contributions of multiple genes.
A few limitations of the current study should be noted. First, although our results add to the accumulating yet complicated picture of the effect of the COMT gene on personality, more replications with different study designs and populations are needed. Second, although we had a decent sample size, the genotype frequency yielded a small Met/Met group. Third, although we used multiple measures and showed consistent results both at the measure and factor levels, it remains to be seen whether there are other measures and factors that should be included as COMT-related personality traits.
In summary, our study overcame some of the weaknesses of the previous research (small samples, ethnic stratification, single measures, lacking of attention to sex differences) and reported significant sex–COMT interaction effects on personality. Taken together our results and those of previous studies, it is imperative to include sex in future research on gene–behavior relations and to model gene–gene and gene–environment interactions.
References
Barnett JH, Scoriels L, Munafo MR (2008). Meta-analysis of the cognitive effects of the catechol-O-methyltransferase gene Val158/108Met polymorphism. Biol Psychiatry 64: 137–144.
Beck A, Steer R (1990). Manual for the Beck Anxiety Inventory. Psychological Corporation: San Antonio, TX.
Beck A, Steer R, Brown G (1996). The Beck Depression Inventory—Second Edition Manual. The Psychological Corporation: San Antonio, TX.
Benjamin J, Osher Y, Kotler M, Gritsenko I, Nemanov L, Belmaker RH et al (2000). Association between tridimensional personality questionnaire (TPQ) traits and three functional polymorphisms: dopamine receptor D4 (DRD4), serotonin transporter promoter region (5-HTTLPR) and catechol O-methyltransferase (COMT). Mol Psychiatry 5: 96–100.
Beuten J, Payne T, Ma J, Li M (2006). Significant association of catechol-O-methyltransferase (COMT) haplotypes with nicotine dependence in male and female smokers of two ethnic populations. Neuropsychopharmacology 31: 675–684.
Bouchard TJ (1994). Genes, environment, and personality. Science 264: 1700–1701.
Bouchard TJ, Loehlin JC (2001). Genes, evolution, and personality. Behav Genet 31: 243–273.
Briggs MH, Briggs M (1973). Hormonal influences on erythrocyte catechol-O-methyl transferase activity in humans. Experientia 29: 278–280.
Calati R, Porcelli S, Giegling I, Hartmann AM, Moller HJ, De Ronchi D et al (2011). Catechol-o-methyltransferase gene modulation on suicidal behavior and personality traits: review, meta-analysis and association study. J Psychiatr Res 45: 309–321.
Carver CS, White TL (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS BAS scales. J Pers Soc Psychol 67: 319–333.
Chen JS, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S et al (2004). Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet 75: 807–821.
Cloninger CR, Svrakic DM, Przybeck TR (1993). A psychobiological model of temperament and character. Arch Gen Psychiatry 50: 975–990.
Cloninger CR, Svrakic DM, Przybeck TR (2006). Can personality assessment predict future depression? A twelve-month follow-up of 631 subjects. J Affect Disord 92: 35–44.
Costa PT, McCrae RL (1992). Revised NEO Personality Inventory and NEO Five-Factor Inventory: Professional Manual. Psychological Assessment Resources: Odessa, FL.
Creveling CR (2003). The role of catechol-O-methyltransferase in the inactivation of catecholestrogen. Cell Mol Neurobiol 23: 289–291.
Darcan A, Onur E, Kose T, Alkin T, Erdem A (2008). Character and temperament dimensions of patients with temporomandibular disorder. Turk Psikiyatri Derg 19: 274–282.
Demetrovics Z, Varga G, Szekely A, Vereczkei A, Csorba J, Balazs H et al (2010). Association between Novelty Seeking of opiate-dependent patients and the catechol-O-methyltransferase Val(158)Met polymorphism. Compr Psychiatry 51: 510–515.
Dmitrieva J, Chen C, Greenberger E, Ogunseitan D, Ding Y (2011). Gender-specific expression of the DRD4 gene on adolescent delinquency, anger and thrill seeking. Soc Cog Affect Neurosci 6: 82–89.
Domschke K, Deckert J, O’Donovan MC, Glatt SJ (2007). Metaanalysis of COMT val158met in panic disorder: ethnic heterogeneity and gender specificity. Am J Med Genet B Neuropsychiatr Genet 144B: 667–673.
Eley TC, Tahir E, Angleitner A, Harriss K, McClay J, Plomin R et al (2003). Association analysis of MAOA and COMT with neuroticism assessed by peers. Am J Med Genet B Neuropsychiatr Genet 120B: 90–96.
Enoch MA, Xu K, Ferro E, Harris CR, Goldman D (2003). Genetic origins of anxiety in women: a role for a functional catechol-O-methyltransferase polymorphism. Psychiatr Genet 13: 33–41.
Goldman D, Weinberger DR, Malhotra AK, Goldberg TE (2009). The role of COMT Val158Met in cognition. Biol Psychiatry 65: e1–e2; author reply e3–4.
Golimbet VE, Alfimova MV, Gritsenko IK, Ebstein RP (2007). Relationship between dopamine system genes and extraversion and novelty seeking. Neurosci Behav Physiol 37: 601–606.
Harrison PJ, Tunbridge EM (2008). Catechol-O-methyltransferase (COMT): a gene contributing to sex differences in brain function, and to sexual dimorphism in the predisposition to psychiatric disorders. Neuropsychopharmacology 33: 3037–3045.
Hashimoto R, Noguchi H, Hori H, Ohi K, Yasuda Y, Takeda M et al (2007). A possible association between the Val158Met polymorphism of the catechol-O-methyl transferase gene and the personality trait of harm avoidance in Japanese healthy subjects. Neurosci Lett 428: 17–20.
Heiman N, Stallings MC, Young SE, Hewitt JK (2004). Investigating the genetic and environmental structure of Cloninger's personality dimensions in adolescence. Twins Res 7: 462–470.
Hoth KF, Paul RH, Williams LM, Dobson-Stone C, Todd E, Schofield PR et al (2006). Associations between the COMT Val/Met polymorphism, early life stress, and personality among healthy adults. Neuropsychiatr Dis Treat 2: 219–225.
Ishii G, Suzuki A, Oshino S, Shiraishi H, Matsumoto Y, Otani K et al (2007). Association study of catechol-O-methyltransferase Val158Met polymorphism with personality traits in Japanese healthy volunteers. Eur Psychiatry 22: 462–465.
Jiang H, Xie T, Ramsden DB, Ho SL (2003). Human catechol-O-methyltransferase down-regulation by estradiol. Neuropharmacology 45: 1011–1018.
Kang JI, Song DH, Namkoong K, Kim SJ (2010). Interaction effects between COMT and BDNF polymorphisms on boredom susceptibility of sensation seeking traits. Psychiatry Res 178: 132–136.
Kim SJ, Kim YS, Kim SY, Lee HS, Kim CH (2006). An association study of catechol-O-methyltransferase and monoamine oxidase A polymorphisms and personality traits in Koreans. Neurosci Lett 401: 154–158.
Lang UE, Bajbouj M, Sander T, Gallinat J (2007). Gender-dependent association of the functional catechol-O-methyltransferase Val158Met genotype with sensation seeking personality trait. Neuropsychopharmacology 32: 1950–1955.
Light KJ, Joyce PR, Luty SE, Mulder RT, Carter JD, Frampton M et al (2007). An association study of DRD2 and COMT polymorphisms with novelty seeking and harm avoidance scores, in two independent samples of depressed patients. Behav Brain Funct 3: 3.
Lin CC, Su CH, Kuo PH, Hsiao CK, Soong WT, Chen WJ (2007). Genetic and environmental influences on schizotypy among adolescents in Taiwan: a multivariate twin/sibling Analysis. Behav Genet 37: 334–344.
Long AD, Langley CH (1999). The power of association studies to detect the contribution of candidate genetic loci to variation in complex traits. Genome Res 9: 720–731.
Munafo MR, Clark TG, Moore LR, Payne E, Walton R, Flint J (2003). Genetic polymorphisms and personality in healthy adults: a systematic review and meta-analysis. Mol Psychiatry 8: 471–484.
O’Hara R, Miller E, Liao CP, Way N, Lin XY, Hallmayer J (2006). COMT genotype, gender and cognition in community-dwelling, older adults. Neurosci Lett 409: 205–209.
Olsson CA, Anney RJ, Lotfi-Miri M, Byrnes GB, Williamson R, Patton GC (2005). Association between the COMT Val158Met polymorphism and propensity to anxiety in an Australian population-based longitudinal study of adolescent health. Psychiatr Genet 15: 109–115.
Qian Q, Wang Y, Zhou R, Li J, Wang B, Glatt S et al (2003). Family-based and case-control association studies of catechol-O-methyltransferase in attention deficit hyperactivity disorder suggest genetic sexual dimorphism. Am J Med Genet B Neuropsychiatr Genet 118B: 103–109.
Reuter M, Hennig J (2005). Association of the functional catechol-O-methyltransferase VAL158MET polymorphism with the personality trait of extraversion. Neuroreport 16: 1135–1138.
Reuter M, Kirsch P, Hennig J (2006a). Inferring candidate genes for attention deficit hyperactivity disorder (ADHD) assessed by the World Health Organization Adult ADHD Self-Report Scale (ASRS). J Neural Transm 113: 929–938.
Reuter M, Schmitz A, Corr P, Hennig J (2006b). Molecular genetics support Gray's personality theory: the interaction of COMT and DRD2 polymorphisms predicts the behavioural approach system. Int J Neuropsychopharmacol 9: 155–166.
Rujescu D, Giegling I, Gietl A, Hartmann AM, Moller HJ (2003). A functional single nucleotide polymorphism (V158 M) in the COMT gene is associated with aggressive personality traits. Biol Psychiatry 54: 34–39.
Salo J, Pulkki-Raback L, Hintsanen M, Lehtimaki T, Keltikangas-Jarvinen L (2010). The interaction between serotonin receptor 2A and catechol-O-methyltransferase gene polymorphisms is associated with the novelty-seeking subscale impulsiveness. Psychiatr Genet 20: 273–281.
Savitz J, Solms M, Ramesar R (2006). The molecular genetics of cognition: dopamine, COMT and BDNF. Genes Brain Behav 5: 311–328.
Schmitt DP, Realo A, Voracek M, Allik J (2008). Why can′t a man be more like a woman? Sex differences in Big Five personality traits across 55 cultures. J Pers Soc Psychol 94: 168–182.
Sheldrick AJ, Krug A, Markov V, Leube D, Michel TM, Zerres K et al (2008). Effect of COMT val158met genotype on cognition and personality. Eur Psychiatry 23: 385–389.
Stein DJ, Newman TK, Savitz J, Ramesar R (2006). Warriors versus worriers: the role of COMT gene variants. CNS Spectr 11: 745–748.
Stein MB, Fallin MD, Schork NJ, Gelernter J (2005). COMT polymorphisms and anxiety-related personality traits. Neuropsychopharmacology 30: 2092–2102.
Strobel A, Lesch KP, Jatzke S, Paetzold F, Brocke B (2003). Further evidence for a modulation of Novelty Seeking by DRD4 exon III, 5-HTTLPR, and COMT val/met variants. Mol Psychiatry 8: 371–372.
Tochigi M, Otowa T, Hibino H, Kato C, Otani T, Umekage T et al (2006). Combined analysis of association between personality traits and three functional polymorphisms in the tyrosine hydroxylase, monoamine oxidase A, and catechol-O-methyltransferase genes. Neurosci Res 54: 180–185.
Tsai SJ, Hong CJ, Yu YW, Chen TJ (2004). Association study of catechol-O-methyltransferase gene and dopamine D4 receptor gene polymorphisms and personality traits in healthy young chinese females. Neuropsychobiology 50: 153–156.
Tunbridge EM, Weickert CS, Kleinman JE, Herman MM, Chen J, Kolachana BS et al (2007). Catechol-O-methyltransferase enzyme activity and protein expression in human prefrontal cortex across the postnatal lifespan. Cereb Cortex 17: 1206–1212.
Urata T, Takahashi N, Hakamata Y, Iijima Y, Kuwahara N, Ozaki N et al (2007). Gene-gene interaction analysis of personality traits in a Japanese population using an electrochemical DNA array chip analysis. Neurosci Lett 414: 209–212.
Wacker J, Gatt JM (2010). Resting posterior versus frontal delta/theta EEG activity is associated with extraversion and the COMT VAL(158)MET polymorphism. Neurosci Lett 478: 88–92.
Xie T, Ho SL, Ramsden D (1999). Characterization and implications of estrogenic down-regulation of human catechol-O-methyltransferase gene transcription. Mol Pharmacol 56: 31–38.
Acknowledgements
This study was supported by the 111 Project of the Ministry of Education of China (B07008). We thank all graduate research assistants who helped us with data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
PowerPoint slides
Rights and permissions
About this article
Cite this article
Chen, C., Chen, C., Moyzis, R. et al. Sex Modulates the Associations Between the COMT Gene and Personality Traits. Neuropsychopharmacol 36, 1593–1598 (2011). https://doi.org/10.1038/npp.2011.39
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2011.39
Keywords
This article is cited by
-
Quantitative behavioral genetic and molecular genetic foundations of the approach and avoidance strategies
Current Psychology (2023)
-
Sexually divergent effect of COMT Val/met genotype on subcortical volumes in schizophrenia
Brain Imaging and Behavior (2018)
-
Novelty Seeking and Drug Addiction in Humans and Animals: From Behavior to Molecules
Journal of Neuroimmune Pharmacology (2016)
-
Network-Dependent Modulation of COMT and DRD2 Polymorphisms in Healthy Young Adults
Scientific Reports (2015)
-
The psychiatric vulnerability gene CACNA1C and its sex-specific relationship with personality traits, resilience factors and depressive symptoms in the general population
Molecular Psychiatry (2013)