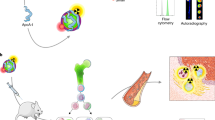
Abstract
In cancer imaging, nanoparticle biodistribution is typically visualized in living subjects using ‘bulk’ imaging modalities such as magnetic resonance imaging, computerized tomography and whole-body fluorescence. Accordingly, nanoparticle influx is observed only macroscopically, and the mechanisms by which they target cancer remain elusive. Nanoparticles are assumed to accumulate via several targeting mechanisms, particularly extravasation (leakage into tumour). Here, we show that, in addition to conventional nanoparticle-uptake mechanisms, single-walled carbon nanotubes are almost exclusively taken up by a single immune cell subset, Ly-6Chi monocytes (almost 100% uptake in Ly-6Chi monocytes, below 3% in all other circulating cells), and delivered to the tumour in mice. We also demonstrate that a targeting ligand (RGD) conjugated to nanotubes significantly enhances the number of single-walled carbon nanotube-loaded monocytes reaching the tumour (P < 0.001, day 7 post-injection). The remarkable selectivity of this tumour-targeting mechanism demonstrates an advanced immune-based delivery strategy for enhancing specific tumour delivery with substantial penetration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Maeda, H. The link between infection and cancer: tumor vasculature, free radicals, and drug delivery to tumors via the EPR effect. Cancer Sci. 7, 779–789 (2013).
Hirsjarvi, S., Passirani, C. & Benoit, J. P. Passive and active tumour targeting with nanocarriers. Curr. Drug Disc. Technol. 8, 188–196 (2011).
Holgado, M. A., Martin-Banderas, L., Alvarez-Fuentes, J., Fernandez-Arevalo, M. & Arias, J. L. Drug targeting to cancer by nanoparticles surface functionalized with special biomolecules. Curr. Med. Chem. 19, 3188–3195 (2012).
Yu, M. K., Park, J. & Jon, S. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics 2, 3–44 (2012).
Liu, Z. et al. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nature Nanotech. 2, 47–52 (2007).
Hahn, M. A., Singh, A. K., Sharma, P., Brown, S. C. & Moudgil, B. M. Nanoparticles as contrast agents for in-vivo bioimaging: current status and future perspectives. Anal. Bioanal. Chem. 399, 3–27 (2011).
Smith, B. R. et al. High-resolution, serial intravital microscopic imaging of nanoparticle delivery and targeting in a small animal tumor model. Nano Today 8, 126–137 (2013).
Smith, B. R. et al. Shape matters: intravital microscopy reveals surprising geometrical dependence for nanoparticles in tumor models of extravasation. Nano Lett. 12, 3369–3377 (2012).
Kam, N. W., Liu, Z. & Dai, H. Carbon nanotubes as intracellular transporters for proteins and DNA: an investigation of the uptake mechanism and pathway. Angew. Chem. Int Ed. 45, 577–581 (2006).
Lacerda, L. et al. Translocation mechanisms of chemically functionalised carbon nanotubes across plasma membranes. Biomaterials 33, 3334–3343 (2012).
Al-Jamal, K. T. et al. Cellular uptake mechanisms of functionalised multi-walled carbon nanotubes by 3D electron tomography imaging. Nanoscale 3, 2627–2635 (2011).
Moghimi, S. M. et al. Particulate systems for targeting of macrophages: basic and therapeutic concepts. J. Innate Immun. 4, 509–528 (2012).
Porter, A. E. et al. Direct imaging of single-walled carbon nanotubes in cells. Nature Nanotech. 2, 713–717 (2007).
Smith, B. R. et al. Real-time intravital imaging of RGD–quantum dot binding to luminal endothelium in mouse tumor neovasculature. Nano Lett. 8, 2599–2606 (2008).
Smith, B. R., Cheng, Z., De, A., Rosenberg, J. & Gambhir, S. S. Dynamic visualization of RGD–quantum dot binding to tumor neovasculature and extravasation in multiple living mouse models using intravital microscopy. Small 6, 2222–2229 (2010).
Leimgruber, A. et al. Behavior of endogenous tumor-associated macrophages assessed in vivo using a functionalized nanoparticle. Neoplasia 11, 459–468 (2009).
Prabhakar, U. et al. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 73, 2412–2417 (2013).
Choi, M. R. et al. A cellular trojan horse for delivery of therapeutic nanoparticles into tumors. Nano Lett. 7, 3759–3765 (2007).
Owen, M. R. et al. Mathematical modeling predicts synergistic antitumor effects of combining a macrophage-based, hypoxia-targeted gene therapy with chemotherapy. Cancer Res. 71, 2826–2837 (2011).
Murdoch, C., Giannoudis, A. & Lewis, C. E. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood 104, 2224–2234 (2004).
Smith, B. R. et al. Localization to atherosclerotic plaque and biodistribution of biochemically derivatized superparamagnetic iron oxide nanoparticles (SPIONs) contrast particles for magnetic resonance imaging (MRI). Biomed. Microdev. 9, 719–727 (2007).
Lacerda, L., Raffa, S., Prato, M., Bianco, A. & Kostarelos, K. Cell-penetrating CNTs for delivery of therapeutics. Nano Today 2, 38–43 (2007).
Yeste, A., Nadeau, M., Burns, E. J., Weiner, H. L. & Quintana, F. J. Nanoparticle-mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA 109, 11270–11275 (2012).
Cubillos-Ruiz, J. R. et al. Polyethylenimine-based siRNA nanocomplexes reprogram tumor-associated dendritic cells via TLR5 to elicit therapeutic antitumor immunity. J. Clin. Invest. 119, 2231–2244 (2009).
Leuschner, F. et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nature Biotechnol. 29, 1005–1010 (2011).
Roy, A., Singh, M. S., Upadhyay, P. & Bhaskar, S. Combined chemo-immunotherapy as a prospective strategy to combat cancer: a nanoparticle based approach. Mol. Pharmacol. 7, 1778–88 (2010).
Beatty, G. L. et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 331, 1612–1616 (2011).
Movahedi, K. et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 70, 5728–5739 (2010).
Gu, L. et al. Multivalent porous silicon nanoparticles enhance the immune activation potency of agonistic CD40 antibody. Adv. Mater. 24, 3981–3987 (2012).
Elamanchili, P., Diwan, M., Cao, M. & Samuel, J. Characterization of poly(D,L-lactic-co-glycolic acid) based nanoparticulate system for enhanced delivery of antigens to dendritic cells. Vaccine 22, 2406–2412 (2004).
Cruz, L. J. et al. Targeting nanosystems to human DCs via Fc receptor as an effective strategy to deliver antigen for immunotherapy. Mol. Pharmacol. 8, 104–116 (2011).
Cruz, L. J. et al. Multimodal imaging of nanovaccine carriers targeted to human dendritic cells. Mol. Pharmacol. 8, 520–531 (2011).
Gunn, J. et al. A multimodal targeting nanoparticle for selectively labeling T cells. Small 4, 712–715 (2008).
Saha, P. & Geissmann, F. Toward a functional characterization of blood monocytes. Immunol. Cell Biol. 89, 2–4 (2011).
Yona, S. & Jung, S. Monocytes: subsets, origins, fates and functions. Curr. Opin. Hematol. 17, 53–59 (2010).
Shi, C. & Pamer, E. G. Monocyte recruitment during infection and inflammation. Nature Rev. Immunol. 11, 762–774 (2011).
Primeau, A. J., Rendon, A., Hedley, D., Lilge, L. & Tannock, I. F. The distribution of the anticancer drug Doxorubicin in relation to blood vessels in solid tumors. Clin. Cancer Res. 11, 8782–8788 (2005).
Ingersoll, M. A. et al. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood 115, e10–e19 (2010).
De Nicola, M. et al. Effects of carbon nanotubes on human monocytes. Ann. NY Acad. Sci. 1171, 600–605 (2009).
Schipper, M. L. et al. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nature Nanotech. 3, 216–221 (2008).
Lee, H. J. et al. Amine-modified single-walled carbon nanotubes protect neurons from injury in a rat stroke model. Nature Nanotech. 6, 121–125 (2011).
Liu, Z., Tabakman, S. M., Chen, Z. & Dai, H. Preparation of carbon nanotube bioconjugates for biomedical applications. Nature Protoc. 4, 1372–1382 (2009).
Herzenberg, L. A., Tung, J., Moore, W. A. & Parks, D. R. Interpreting flow cytometry data: a guide for the perplexed. Nature Immunol. 7, 681–685 (2006).
Ghosn, E. E. et al. Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proc. Natl Acad. Sci. USA 107, 2568–2573 (2010).
Acknowledgements
This work was supported by a number of grants. B.R.S. was supported by a National Institutes of Health (NIH) 25T post-doctoral training grant and is currently supported by a K99/R00 award (K99 CA160764). S.S.G. was supported by a Center for Cancer Nanotechnology Excellence–Translation (CCNE-T)-grant (National Cancer Institute U54 CA119367) and in vivo cellular and molecular imaging (ICMIC) grant (P50 CA114747). The authors thank C. Ball for discussions related to this work. The authors acknowledge technical assistance in experiments and analysis from M. Philips, S. Tabakman, J. Rosenberg, S. Kusy, C. Nielsen, H. Dai, A-L. Koh, R. Sinclair, C. Zavaleta, J. Strommer and CytoViva.
Author information
Authors and Affiliations
Contributions
B.R.S. and S.S.G. conceived and designed the experiments. B.R.S. performed the experiments and contributed materials and analysis tools. B.R.S. and S.S.G. analysed the data and wrote the manuscript. E.E.B.G. designed, performed and analysed 12-colour, 14-parameter FACS for immune cell analysis of blood samples, discussed results, contributed reagents, and commented on the manuscript. L.A.H. provided guidance and assisted with analysis regarding high-dimensional flow cytometry and contributed materials and analysis tools. H.R. assisted with analyses of intravital imaging experiments. B.R.S. and J.A.P. designed, performed and analysed initial flow cytometry assays. T.L. assisted with characterization of nanotubes.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 5760 kb)
Supplementary movie 1
Supplementary movie 1 (MOV 939 kb)
Supplementary movie 2
Supplementary movie 2 (MOV 28133 kb)
Supplementary movie 3
Supplementary movie 3 (MOV 877 kb)
Supplementary movie 4
Supplementary movie 4 (MOV 1466 kb)
Rights and permissions
About this article
Cite this article
Smith, B., Ghosn, E., Rallapalli, H. et al. Selective uptake of single-walled carbon nanotubes by circulating monocytes for enhanced tumour delivery. Nature Nanotech 9, 481–487 (2014). https://doi.org/10.1038/nnano.2014.62
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2014.62
This article is cited by
-
Nanomedicines for cardiovascular disease
Nature Cardiovascular Research (2023)
-
Biomedical polymers: synthesis, properties, and applications
Science China Chemistry (2022)
-
Recent advances in selective photothermal therapy of tumor
Journal of Nanobiotechnology (2021)
-
Nanotherapeutics for cardiovascular disease
Nature Reviews Cardiology (2021)
-
Host responses to implants revealed by intravital microscopy
Nature Reviews Materials (2021)