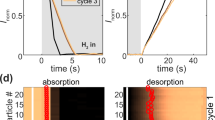
Abstract
Many energy- and information-storage processes rely on phase changes of nanomaterials in reactive environments. Compared to their bulk counterparts, nanostructured materials seem to exhibit faster charging and discharging kinetics, extended life cycles, and size-tunable thermodynamics. However, in ensemble studies of these materials, it is often difficult to discriminate between intrinsic size-dependent properties and effects due to sample size and shape dispersity. Here, we detect the phase transitions of individual palladium nanocrystals during hydrogen absorption and desorption, using in situ electron energy-loss spectroscopy in an environmental transmission electron microscope. In contrast to ensemble measurements, we find that palladium nanocrystals undergo sharp transitions between the α and β phases, and that surface effects dictate the size dependence of the hydrogen absorption pressures. Our results provide a general framework for monitoring phase transitions in individual nanocrystals in a reactive environment and highlight the importance of single-particle approaches for the characterization of nanostructured materials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Pundt, A. Hydrogen in nano-sized metals. Adv. Eng. Mater. 6, 11–21 (2004).
Bérubé, V., Radtke, G., Dresselhaus, M. & Chen, G. Size effects on the hydrogen storage properties of nanostructured metal hydrides: A review. Int. J. Energy Res. 31, 637–663 (2007).
Bardhan, R. et al. Uncovering the intrinsic size dependence of hydriding phase transformations in nanocrystals. Nature Mater. 12, 905–912 (2013).
Aricò, A. S., Bruce, P., Scrosati, B., Tarascon, J-M. & van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nature Mater. 4, 366–377 (2005).
Meethong, N., Huang, H-Y. S., Speakman, S. A., Carter, W. C. & Chiang, Y-M. Strain accommodation during phase transformations in olivine-based cathodes as a materials selection criterion for high-power rechargeable batteries. Adv. Funct. Mater. 17, 1115–1123 (2007).
Bruce, P. G., Scrosati, B. & Tarascon, J-M. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. 47, 2930–2946 (2008).
Chueh, W. C. et al. Intercalation pathway in many-particle LiFePO4 electrode revealed by nanoscale state-of-charge mapping. Nano Lett. 13, 866–872 (2013).
Ebner, M., Marone, F., Stampanoni, M. & Wood, V. Visualization and quantification of electrochemical and mechanical degradation in Li ion batteries. Science 342, 716–720 (2013).
Jeong, J. et al. Suppression of metal–insulator transition in VO2 by electric field-induced oxygen vacancy formation. Science 339, 1402–1405 (2013).
Ohno, T. et al. Short-term plasticity and long-term potentiation mimicked in single inorganic synapses. Nature Mater. 10, 591–595 (2011).
Son, D. H., Hughes, S. M., Yin, Y. & Alivisatos, A. P. Cation exchange reactions in ionic nanocrystals. Science 306, 1009–1012 (2004).
Graham, T. On the absorption and dialytic separation of gases by colloid septa. Phil. Trans. R. Soc. Lond. 156, 399–439 (1866).
Sachs, C. et al. Solubility of hydrogen in single-sized palladium clusters. Phys. Rev. B 64, 075408 (2001).
Pundt, A. & Kirchheim, R. Hydrogen in metals: Microstructural aspects. Annu. Rev. Mater. Res. 36, 555–608 (2006).
Yamauchi, M., Ikeda, R., Kitagawa, H. & Takata, M. Nanosize effects on hydrogen storage in palladium. J. Phys. Chem. C 112, 3294–3299 (2008).
Zheng, H. et al. Observation of transient structural-transformation dynamics in a Cu2S nanorod. Science 333, 206–209 (2011).
Routzahn, A. L. & Jain, P. K. Single-nanocrystal reaction trajectories reveal sharp cooperative transitions. Nano Lett. 12, 987–992 (2014).
Liu, N., Tang, M. L., Hentschel, M., Giessen, H. & Alivisatos, A. P. Nanoantenna-enhanced gas sensing in a single tailored nanofocus. Nature Mater. 10, 631–636 (2011).
Shegai, T. & Langhammer, C. Hydride formation in single palladium and magnesium nanoparticles studied by nanoplasmonic dark-field scattering spectroscopy. Adv. Mater. 23, 4409–4414 (2011).
Tang, M. L., Liu, N., Dionne, J. A. & Alivisatos, A. P. Observations of shape-dependent hydrogen uptake trajectories from single nanocrystals. J. Am. Chem. Soc. 133, 13220–13223 (2011).
Tittl, A., Kremers, C., Dorfmüller, J., Chigrin, D. N. & Giessen, H. Spectral shifts in optical nanoantenna-enhanced hydrogen sensors. Opt. Mater. Express 2, 111–118 (2012).
García de Abajo, F. J. Optical excitations in electron microscopy. Rev. Mod. Phys. 82, 209–275 (2010).
Gremaud, R., Slaman, M., Schreuders, H., Dam, B. & Griessen, R. An optical method to determine the thermodynamics of hydrogen absorption and desorption in metals. Appl. Phys. Lett. 91, 231916 (2007).
Bennett, P. A. & Fuggle, J. C. Electronic structure and surface kinetics of palladium hydride studied with x-ray photoelectron spectroscopy and electron-energy-loss spectroscopy. Phys. Rev. B 26, 6030–6039 (1982).
Liu, D. R. & Brown, L. M. Characterization of palladium hydride films by electron energy loss spectroscopy and electron diffraction. Acta Metall. 36, 2597–2604 (1988).
Niu, W. et al. Seed-mediated growth of nearly monodisperse palladium nanocubes with controllable sizes. Cryst. Growth Des. 8, 4440–4444 (2008).
Pundt, A. et al. Hydrogen sorption in elastically soft stabilized Pd-clusters. J. Alloys Compd 293–295, 480–483 (1999).
Schwarz, R. B. & Khachaturyan, A. G. Thermodynamics of open two-phase systems with coherent interfaces: Application to metal-hydrogen systems. Acta Mater. 54, 313–323 (2006).
Scholl, J. A., Koh, A. L. & Dionne, J. A. Quantum plasmon resonances of individual metallic nanoparticles. Nature 483, 421–427 (2012).
Jung, H. J. et al. Spatial variation of available electronic excitations within individual quantum dots. Nano Lett. 13, 716–721 (2013).
Wagner, H. in Topics in Applied Physics: Hydrogen in Metals I (eds Alefeld, G. & Völkl, J.) (Springer, 1978).
Nörthemann, K. & Pundt, A. Coherent-to-semi-coherent transition of precipitates in niobium-hydrogen thin films. Phys. Rev. B 78, 014105 (2008).
Wagner, S. et al. Achieving coherent phase transition in palladium-hydrogen thin films. Scr. Mater. 64, 978–981 (2011).
Langhammer, C., Zhdanov, V. P., Zorić, I. & Kasemo, B. Size-dependent kinetics of hydriding and dehydriding of Pd nanoparticles. Phys. Rev. Lett. 104, 135502 (2010).
Wagemaker, M., Borghols, W. J. H. & Mulder, F. M. Large impact of particle size on insertion reactions. A case for anatase LixTiO2 . J. Am. Chem. Soc. 129, 4323–4327 (2007).
Liu, H. et al. Capturing metastable structures during high-rate cycling of LiFePO4 nanoparticle electrodes. Science 344, 1252817 (2014).
Weissmüller, J. & Lemier, C. On the size dependence of the critical point of nanoscale interstitial solid solutions. Phil. Mag. Lett. 80, 411–418 (2000).
Lemier, C. & Weissmüller, J. Grain boundary segregation, stress and stretch: Effects on hydrogen absorption in nanocrystalline palladium. Acta Mater. 55, 1241–1254 (2007).
Mütschele, T. & Kirchheim, R. Segregation and diffusion of hydrogen in grain boundaries of palladium. Scr. Metall. 21, 135–140 (1987).
Mütschele, T. & Kirchheim, R. Hydrogen as a probe for the average thickness of a grain boundary. Scr. Metall. 21, 1101–1104 (1987).
Griessen, R. & Feenstra, R. Volume changes during hydrogen absorption in metals. J. Phys. F 15, 1013–1019 (1985).
Brodowsky, H. On the non-ideal solution behavior of hydrogen in metals. Ber. Bunsenges. Phys. Chem. 76, 740–746 (1972).
Andreev, A. D., Downes, J. R., Faux, D. A. & O’Reilly, E. P. Strain distributions in quantum dots of arbitrary shape. J. Appl. Phys. 86, 297–305 (1999).
Rockenberger, J. et al. The contribution of particle core and surface to strain, disorder and vibrations in thiolcapped CdTe nanocrystals. J. Chem. Phys. 108, 7807–7815 (1998).
Fahmy, A. A. & Ragay, A. N. Thermal-expansion behavior of two-phase solids. J. Appl. Phys. 41, 5108–5111 (1970).
Hsu, D. K. & Leisure, R. G. Elastic constants of palladium and β-phase palladium hydride between 4 and 300 K. Phys. Rev. B 20, 1339–1344 (1979).
Wilde, M., Matsumoto, M., Fukutani, K. & Aruga, T. Depth-resolved analysis of subsurface hydrogen absorbed by Pd(100). Surf. Sci. 482–485, 346–352 (2001).
Clewley, J. D., Curran, T., Flanagan, T. B. & Oates, W. A. Thermodynamic properties of hydrogen and deuterium dissolved in palladium at low concentrations over a wide temperature range. J. Chem. Soc. Faraday Trans. 1 69, 449–458 (1972).
Wicke, E. & Brodowsky, H. in Topics in Applied Physics: Hydrogen in Metals II (eds Alefeld, G. & Völkl, J.) (Springer, 1978).
Acknowledgements
We gratefully acknowledge scientific feedback and discussions with J. Scholl, W. D. Nix, R. Griessen and A. Pundt. J.A.D. acknowledges support from a Stanford Terman Fellowship, a Hellman Fellowship, an Air Force Office of Scientific Research Young Investigator Grant (FA9550-11-1-0024) and a National Science Foundation CAREER Award (DMR-1151231). This work was supported in part by a SLAC National Accelerator Laboratory LDRD award in concert with the Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering, under contract DEAC02-76SF00515. Work was also supported by the Young Energy Scientist (YES!) Fellowship of the Foundation for Fundamental Research on Matter (FOM), which is financially supported by the Netherlands Organisation for Scientific Research (NWO), and by an award from the Department of Energy (DOE) Office of Science Graduate Fellowship Program administered by the Oak Ridge Institute for Science and Education for the DOE. ORISE is managed by Oak Ridge Associated Universities (ORAU) under DOE contract number DE-AC05-06OR23100. All opinions expressed in this paper are of the authors and do not necessarily reflect the policies and views of NSF, DOE, ORAU or ORISE.
Author information
Authors and Affiliations
Contributions
A.B., T.C.N. and J.A.D. designed the experiments and A.B., T.C.N. and A.L.K. performed the experiments. A.B. and T.C.N. analysed the data and wrote the initial draft of the manuscript. J.A.D. supervised the project, and all authors discussed the results and contributed to final manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1707 kb)
Rights and permissions
About this article
Cite this article
Baldi, A., Narayan, T., Koh, A. et al. In situ detection of hydrogen-induced phase transitions in individual palladium nanocrystals. Nature Mater 13, 1143–1148 (2014). https://doi.org/10.1038/nmat4086
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4086
This article is cited by
-
Small, solubilized platinum nanocrystals consist of an ordered core surrounded by mobile surface atoms
Communications Chemistry (2024)
-
Naked-eye observation of water-forming reaction on palladium etalon: transduction of gas-matter reaction into light-matter interaction
PhotoniX (2023)
-
On-chip ultrasensitive and rapid hydrogen sensing based on plasmon-induced hot electron–molecule interaction
Light: Science & Applications (2023)
-
Generation of oxide surface patches promoting H-spillover in Ru/(TiOx)MnO catalysts enables CO2 reduction to CO
Nature Catalysis (2023)
-
Breaking scaling relationships in alkynol semi-hydrogenation by manipulating interstitial atoms in Pd with d-electron gain
Nature Communications (2022)