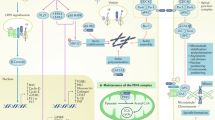
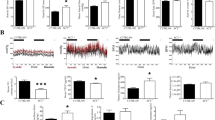
Abstract
Hypertension is the most common cardiovascular disease, afflicting >30% of adults1. The cause of hypertension in most individuals remains unknown2,3, suggesting that additional contributing factors have yet to be discovered. Corin is a serine protease that activates the natriuretic peptides, thereby regulating blood pressure4. It is synthesized as a zymogen that is activated by proteolytic cleavage. CORIN variants and mutations impairing corin activation have been identified in people with hypertension and pre-eclampsia5,6,7,8,9. To date, however, the identity of the protease that activates corin remains elusive. Here we show that proprotein convertase subtilisin/kexin-6 (PCSK6, also named PACE4; ref. 10) cleaves and activates corin. In cultured cells, we found that corin activation was inhibited by inhibitors of PCSK family proteases and by small interfering RNAs blocking PCSK6 expression. Conversely, PCSK6 overexpression enhanced corin activation. In addition, purified PCSK6 cleaved wild-type corin but not the R801A variant that lacks the conserved activation site. Pcsk6-knockout mice developed salt-sensitive hypertension, and corin activation and pro-atrial natriuretic peptide processing activity were undetectable in these mice. Moreover, we found that CORIN variants in individuals with hypertension and pre-eclampsia were defective in PCSK6-mediated activation. We also identified a PCSK6 mutation that impaired corin activation activity in a hypertensive patient. Our results indicate that PCSK6 is the long-sought corin activator and is important for sodium homeostasis and normal blood pressure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Go, A.S. et al. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation 127, e6–e245 (2013).
Lifton, R.P., Gharavi, A.G. & Geller, D.S. Molecular mechanisms of human hypertension. Cell 104, 545–556 (2001).
Oparil, S., Zaman, M.A. & Calhoun, D.A. Pathogenesis of hypertension. Ann. Intern. Med. 139, 761–776 (2003).
Yan, W., Wu, F., Morser, J. & Wu, Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc. Natl. Acad. Sci. USA 97, 8525–8529 (2000).
Cui, Y. et al. Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy. Nature 484, 246–250 (2012).
Dong, N. et al. Corin mutation R539C from hypertensive patients impairs zymogen activation and generates an inactive alternative ectodomain fragment. J. Biol. Chem. 288, 7867–7874 (2013).
Dries, D.L. et al. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation 112, 2403–2410 (2005).
Rame, J.E. et al. Corin I555(P568) allele is associated with enhanced cardiac hypertrophic response to increased systemic afterload. Hypertension 49, 857–864 (2007).
Wang, W. et al. Corin variant associated with hypertension and cardiac hypertrophy exhibits impaired zymogen activation and natriuretic peptide processing activity. Circ. Res. 103, 502–508 (2008).
Seidah, N.G., Sadr, M.S., Chretien, M. & Mbikay, M. The multifaceted proprotein convertases: their unique, redundant, complementary, and opposite functions. J. Biol. Chem. 288, 21473–21481 (2013).
Guyton, A.C. Blood pressure control–special role of the kidneys and body fluids. Science 252, 1813–1816 (1991).
Mullins, L.J., Bailey, M.A. & Mullins, J.J. Hypertension, kidney, and transgenics: a fresh perspective. Physiol. Rev. 86, 709–746 (2006).
Cowley, A.W. Jr. The genetic dissection of essential hypertension. Nat. Rev. Genet. 7, 829–840 (2006).
McGrath, M.F., de Bold, M.L. & de Bold, A.J. The endocrine function of the heart. Trends Endocrinol. Metab. 16, 469–477 (2005).
Newton-Cheh, C. et al. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat. Genet. 41, 348–353 (2009).
Barbato, E. et al. Influence of rs5065 atrial natriuretic peptide gene variant on coronary artery disease. J. Am. Coll. Cardiol. 59, 1763–1770 (2012).
Cannone, V. et al. Atrial natriuretic peptide genetic variant rs5065 and risk for cardiovascular disease in the general community: a 9-year follow-up study. Hypertension 62, 860–865 (2013).
Hodgson-Zingman, D.M. et al. Atrial natriuretic peptide frameshift mutation in familial atrial fibrillation. N. Engl. J. Med. 359, 158–165 (2008).
Lynch, A.I., Claas, S.A. & Arnett, D.K. A review of the role of atrial natriuretic peptide gene polymorphisms in hypertension and its sequelae. Curr. Hypertens. Rep. 11, 35–42 (2009).
Yan, W., Sheng, N., Seto, M., Morser, J. & Wu, Q. Corin, a mosaic transmembrane serine protease encoded by a novel cDNA from human heart. J. Biol. Chem. 274, 14926–14935 (1999).
Jiang, J. et al. Ectodomain shedding and autocleavage of the cardiac membrane protease corin. J. Biol. Chem. 286, 10066–10072 (2011).
Rockwell, N.C., Krysan, D.J., Komiyama, T. & Fuller, R.S. Precursor processing by kex2/furin proteases. Chem. Rev. 102, 4525–4548 (2002).
Beaubien, G. et al. The distinct gene expression of the pro-hormone convertases in the rat heart suggests potential substrates. Cell Tissue Res. 279, 539–549 (1995).
Wang, W. et al. Impaired sodium excretion and salt-sensitive hypertension in corin-deficient mice. Kidney Int. 82, 26–33 (2012).
Constam, D.B. & Robertson, E.J. SPC4/PACE4 regulates a TGFβ signaling network during axis formation. Genes Dev. 14, 1146–1155 (2000).
Wang, W. et al. Salt-sensitive hypertension and cardiac hypertrophy in transgenic mice expressing a corin variant identified in blacks. Hypertension 60, 1352–1358 (2012).
Dong, N. et al. Corin mutations K317E and S472G from preeclamptic patients alter zymogen activation and cell surface targeting. J. Biol. Chem. 289, 17909–17916 (2014).
Taneda, H., Andoh, K., Nishioka, J., Takeya, H. & Suzuki, K. Blood coagulation factor Xa interacts with a linear sequence of the kringle 2 domain of prothrombin. J. Biochem. 116, 589–597 (1994).
Guipponi, M. et al. The transmembrane serine protease (TMPRSS3) mutated in deafness DFNB8/10 activates the epithelial sodium channel (ENaC) in vitro. Hum. Mol. Genet. 11, 2829–2836 (2002).
Chan, J.C. et al. Hypertension in mice lacking the proatrial natriuretic peptide convertase corin. Proc. Natl. Acad. Sci. USA 102, 785–790 (2005).
Chen, H.H. Heart failure: a state of brain natriuretic peptide deficiency or resistance or both!. J. Am. Coll. Cardiol. 49, 1089–1091 (2007).
Chen, S. et al. Protease corin expression and activity in failing hearts. Am. J. Physiol. Heart Circ. Physiol. 299, H1687–H1692 (2010).
Dries, D.L. Process matters: Emerging concepts underlying impaired natriuretic peptide system function in heart failure. Circ Heart Fail 4, 107–110 (2011).
Gladysheva, I.P. et al. Corin overexpression improves cardiac function, heart failure, and survival in mice with dilated cardiomyopathy. Hypertension 61, 327–332 (2013).
Li, J.P. et al. The association between paired basic amino acid cleaving enzyme 4 gene haplotype and diastolic blood pressure. Chin. Med. J. (Engl.) 117, 382–388 (2004).
Cohen, J.C. & Hobbs, H.H. Genetics. Simple genetics for a complex disease. Science 340, 689–690 (2013).
Horton, J.D., Cohen, J.C. & Hobbs, H.H. PCSK9: a convertase that coordinates LDL catabolism. J. Lipid Res. 50 (suppl.), S172–S177 (2009).
Seidah, N.G., Awan, Z., Chretien, M. & Mbikay, M. PCSK9: a key modulator of cardiovascular health. Circ. Res. 114, 1022–1036 (2014).
Davidson, M.H. Dyslipidaemia: PCSK9 antibodies: a dividend of the genomics revolution. Nat. Rev. Cardiol. 10, 618–619 (2013).
Seidah, N.G. & Prat, A. The biology and therapeutic targeting of the proprotein convertases. Nat. Rev. Drug Discov. 11, 367–383 (2012).
Liao, X., Wang, W., Chen, S. & Wu, Q. Role of glycosylation in corin zymogen activation. J. Biol. Chem. 282, 27728–27735 (2007).
Knappe, S., Wu, F., Masikat, M.R., Morser, J. & Wu, Q. Functional analysis of the transmembrane domain and activation cleavage of human corin: design and characterization of a soluble corin. J. Biol. Chem. 278, 52363–52370 (2003).
Wu, F., Yan, W., Pan, J., Morser, J. & Wu, Q. Processing of pro-atrial natriuretic peptide by corin in cardiac myocytes. J. Biol. Chem. 277, 16900–16905 (2002).
Qi, X., Jiang, J., Zhu, M. & Wu, Q. Human corin isoforms with different cytoplasmic tails that alter cell surface targeting. J. Biol. Chem. 286, 20963–20969 (2011).
Dinter, A. & Berger, E.G. Golgi-disturbing agents. Histochem. Cell Biol. 109, 571–590 (1998).
Malfait, A.M. et al. A role for PACE4 in osteoarthritis pain: evidence from human genetic association and null mutant phenotype. Ann. Rheum. Dis. 71, 1042–1048 (2012).
Jiang, J., Pristera, N., Wang, W., Zhang, X. & Wu, Q. Effect of sialylated O-glycans in pro-brain natriuretic peptide stability. Clin. Chem. 56, 959–966 (2010).
Vasudevan, N.T. et al. Gβγ-independent recruitment of G-protein coupled receptor kinase 2 drives tumor necrosis factor α-induced cardiac β-adrenergic receptor dysfunction. Circulation 128, 377–387 (2013).
Dong, N. et al. Plasma-soluble corin in patients with heart failure. Circ Heart Fail 3, 207–211 (2010).
Acknowledgements
We thank M. Yin of the Lerner Imaging Core at the Cleveland Clinic for electron microscopic analysis and W. Claycomb of Louisiana State University Medical Center for HL-1 cells. This work was supported in part by grants from the US National Institutes of Health (R01HL089298, R01HD064634, R01HL126697) (Q.W.), a Rush University Pilot Grant (A.-M.M.), the National Natural Science Foundation of China (81170247, 81370718) (N.D.), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Contributions
S.C., P.C., N.D. and Q.W. designed the study. S.C., P.C., J.P., C.Z. and H.W. performed molecular biology, biochemistry, cell biology and mouse model studies. N.D., T.Z., J.Y. and Y. Zhang studied hypertensive patients, collected blood samples, sequenced PCSK6 exons, and made plasmids expressing corin variants. E.E.M. and S.V.N.P. did echocardiographic analysis in mice. R.E.M. and A.-M.M. provided Pcsk6-KO mice. S.C., Y. Zhou and Q.W. wrote the manuscript. All authors critically read and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–16 & Supplementary Table 1 (PDF 1325 kb)
Rights and permissions
About this article
Cite this article
Chen, S., Cao, P., Dong, N. et al. PCSK6-mediated corin activation is essential for normal blood pressure. Nat Med 21, 1048–1053 (2015). https://doi.org/10.1038/nm.3920
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3920
This article is cited by
-
Genetic and RNA-related molecular markers of trastuzumab-chemotherapy-associated cardiotoxicity in HER2 positive breast cancer: a systematic review
BMC Cancer (2022)
-
The Natriuretic Peptides for Hypertension Treatment
High Blood Pressure & Cardiovascular Prevention (2022)
-
Signature selection analysis reveals candidate genes associated with production traits in Iranian sheep breeds
BMC Veterinary Research (2021)
-
Involvement of Spike Protein, Furin, and ACE2 in SARS-CoV-2-Related Cardiovascular Complications
SN Comprehensive Clinical Medicine (2020)
-
The Transmembrane Serine Protease HAT-like 4 Is Important for Epidermal Barrier Function to Prevent Body Fluid Loss
Scientific Reports (2017)