Abstract
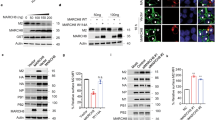
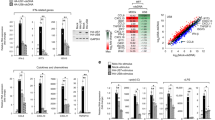
Membrane-associated RING-CH 8 (MARCH8) is one of 11 members of the recently discovered MARCH family of RING (really interesting new gene)-finger E3 ubiquitin ligases1,2. MARCH8 downregulates several host transmembrane proteins, including major histocompatibility complex (MHC)-II, CD86, interleukin (IL)-1 receptor accessory protein, TNF-related apoptosis-inducing ligand (TRAIL) receptor 1 and the transferrin receptor3,4,5,6,7. However, its physiological roles remain largely unknown. Here we identify MARCH8 as a novel antiviral factor. The ectopic expression of MARCH8 in virus-producing cells does not affect levels of lentivirus production, but it does markedly reduce viral infectivity. MARCH8 blocks the incorporation of HIV-1 envelope glycoprotein into virus particles by downregulating it from the cell surface, probably through their interaction, resulting in a substantial reduction in the efficiency of viral entry. The inhibitory effect of MARCH8 on vesicular stomatitis virus G-glycoprotein is even more remarkable, suggesting a broad-spectrum inhibition of enveloped viruses by MARCH8. Notably, the endogenous expression of MARCH8 is high in monocyte-derived macrophages and dendritic cells, and MARCH8 knockdown or knockout in macrophages significantly increases the infectivity of virions produced by these cells. Our findings thus indicate that MARCH8 is highly expressed in terminally differentiated myeloid cells, and that it is a potent antiviral protein that targets viral envelope glycoproteins and reduces their incorporation into virions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bartee, E., Mansouri, M., Hovey Nerenberg, B.T., Gouveia, K. & Fruh, K. Downregulation of major histocompatibility complex class I by human ubiquitin ligases related to viral immune evasion proteins. J. Virol. 78, 1109–1120 (2004).
Goto, E. et al. c-MIR, a human E3 ubiquitin ligase, is a functional homolog of herpesvirus proteins MIR1 and MIR2 and has similar activity. J. Biol. Chem. 278, 14657–14668 (2003).
van de Kooij, B. et al. Ubiquitination by the membrane-associated RING-CH-8 (MARCH-8) ligase controls steady-state cell surface expression of tumor necrosis factor-related apoptosis inducing ligand (TRAIL) receptor 1. J. Biol. Chem. 288, 6617–6628 (2013).
Fujita, H., Iwabu, Y., Tokunaga, K. & Tanaka, Y. Membrane-associated RING-CH (MARCH) 8 mediates the ubiquitination and lysosomal degradation of the transferrin receptor. J. Cell Sci. 126, 2798–2809 (2013).
Chen, R., Li, M., Zhang, Y., Zhou, Q. & Shu, H.B. The E3 ubiquitin ligase MARCH8 negatively regulates IL-1β-induced NF-κB activation by targeting the IL1RAP coreceptor for ubiquitination and degradation. Proc. Natl. Acad. Sci. USA 109, 14128–14133 (2012).
Ohmura-Hoshino, M. et al. Inhibition of MHC class II expression and immune responses by c-MIR. J. Immunol. 177, 341–354 (2006).
Tze, L.E. et al. CD83 increases MHC II and CD86 on dendritic cells by opposing IL-10–driven MARCH1-mediated ubiquitination and degradation. J. Exp. Med. 208, 149–165 (2011).
Ishido, S., Wang, C., Lee, B.S., Cohen, G.B. & Jung, J.U. Downregulation of major histocompatibility complex class I molecules by Kaposi's sarcoma–associated herpesvirus K3 and K5 proteins. J. Virol. 74, 5300–5309 (2000).
Coscoy, L. & Ganem, D. Kaposi's sarcoma–associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis. Proc. Natl. Acad. Sci. USA 97, 8051–8056 (2000).
Stevenson, P.G., Efstathiou, S., Doherty, P.C. & Lehner, P.J. Inhibition of MHC class I-restricted antigen presentation by gamma 2-herpesviruses. Proc. Natl. Acad. Sci. USA 97, 8455–8460 (2000).
Lapaque, N., Jahnke, M., Trowsdale, J. & Kelly, A.P. The HLA-DRα chain is modified by polyubiquitination. J. Biol. Chem. 284, 7007–7016 (2009).
Bartee, E. et al. Membrane-associated RING-CH proteins associate with Bap31 and target CD81 and CD44 to lysosomes. PLoS ONE 5, e15132 (2010).
Eyster, C.A. et al. MARCH ubiquitin ligases alter the itinerary of clathrin-independent cargo from recycling to degradation. Mol. Biol. Cell 22, 3218–3230 (2011).
Harris, R.S. et al. DNA deamination mediates innate immunity to retroviral infection. Cell 113, 803–809 (2003).
Sheehy, A.M., Gaddis, N.C., Choi, J.D. & Malim, M.H. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 418, 646–650 (2002).
Stremlau, M. et al. The cytoplasmic body component TRIM5α restricts HIV-1 infection in Old World monkeys. Nature 427, 848–853 (2004).
Van Damme, N. et al. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3, 245–252 (2008).
Neil, S.J., Zang, T. & Bieniasz, P.D. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451, 425–430 (2008).
Laguette, N. et al. SAMHD1 is the dendritic- and myeloid-cell–specific HIV-1 restriction factor counteracted by Vpx. Nature 474, 654–657 (2011).
Hrecka, K. et al. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature 474, 658–661 (2011).
Liu, Z. et al. The interferon-inducible MxB protein inhibits HIV-1 infection. Cell Host Microbe 14, 398–410 (2013).
Kane, M. et al. MX2 is an interferon-induced inhibitor of HIV-1 infection. Nature 502, 563–566 (2013).
Goujon, C. et al. Human MX2 is an interferon-induced post-entry inhibitor of HIV-1 infection. Nature 502, 559–562 (2013).
Yang, H.C., Shen, L., Siliciano, R.F. & Pomerantz, J.L. Isolation of a cellular factor that can reactivate latent HIV-1 without T cell activation. Proc. Natl. Acad. Sci. USA 106, 6321–6326 (2009).
Jahnke, M., Trowsdale, J. & Kelly, A.P. Structural requirements for recognition of major histocompatibility complex class II by membrane-associated RING-CH (MARCH) protein E3 ligases. J. Biol. Chem. 287, 28779–28789 (2012).
Mashiba, M., Collins, D.R., Terry, V.H. & Collins, K.L. Vpr overcomes macrophage-specific restriction of HIV-1 Env expression and virion production. Cell Host Microbe 16, 722–735 (2014).
Haller, C. et al. HIV-1 Nef and Vpu are functionally redundant broad-spectrum modulators of cell surface receptors, including tetraspanins. J. Virol. 88, 14241–14257 (2014).
Tokarev, A. & Guatelli, J. Misdirection of membrane trafficking by HIV-1 Vpu and Nef: Keys to viral virulence and persistence. Cell. Logist. 1, 90–102 (2011).
Tokunaga, K. et al. Molecular basis for cell tropism of CXCR4-dependent human immunodeficiency virus type 1 isolates. J. Virol. 75, 6776–6785 (2001).
Pham, H.M., Argañaraz, E.R., Groschel, B., Trono, D. & Lama, J. Lentiviral vectors interfering with virus-induced CD4 down-modulation potently block human immunodeficiency virus type 1 replication in primary lymphocytes. J. Virol. 78, 13072–13081 (2004).
Wiznerowicz, M. & Trono, D. Conditional suppression of cellular genes: lentivirus vector-mediated drug-inducible RNA interference. J. Virol. 77, 8957–8961 (2003).
Iwabu, Y. et al. HIV-1 accessory protein Vpu internalizes cell-surface BST-2/tetherin through transmembrane interactions leading to lysosomes. J. Biol. Chem. 284, 35060–35072 (2009).
Tobiume, M., Lineberger, J.E., Lundquist, C.A., Miller, M.D. & Aiken, C. Nef does not affect the efficiency of human immunodeficiency virus type 1 fusion with target cells. J. Virol. 77, 10645–10650 (2003).
Kinomoto, M. et al. Amino acid 36 in the human immunodeficiency virus type 1 gp41 ectodomain controls fusogenic activity: implications for the molecular mechanism of viral escape from a fusion inhibitor. J. Virol. 79, 5996–6004 (2005).
Kinomoto, M. et al. All APOBEC3 family proteins differentially inhibit LINE-1 retrotransposition. Nucleic Acids Res. 35, 2955–2964 (2007).
Daito, T. et al. A novel borna disease virus vector system that stably expresses foreign proteins from an intercistronic noncoding region. J. Virol. 85, 12170–12178 (2011).
Adachi, A. et al. Productive, persistent infection of human colorectal cell lines with human immunodeficiency virus. J. Virol. 61, 209–213 (1987).
Koyanagi, Y. et al. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science 236, 819–822 (1987).
Kitagawa, Y. et al. Inhibitory function of adapter-related protein complex 2 β 1 subunit in the process of nuclear translocation of human immunodeficiency virus type 1 genome. Virology 373, 171–180 (2008).
Mochizuki, N. et al. An infectious DNA clone of HIV type 1 subtype C. AIDS Res. Hum. Retroviruses 15, 1321–1324 (1999).
Shibata, R. et al. Mutational analysis of the human immunodeficiency virus type 2 (HIV-2) genome in relation to HIV-1 and simian immunodeficiency virus SIV (AGM). J. Virol. 64, 742–747 (1990).
Naidu, Y.M. et al. Characterization of infectious molecular clones of simian immunodeficiency virus (SIVmac) and human immunodeficiency virus type 2: persistent infection of rhesus monkeys with molecularly cloned SIVmac. J. Virol. 62, 4691–4696 (1988).
Niwa, H., Yamamura, K. & Miyazaki, J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108, 193–199 (1991).
Fouchier, R.A., Meyer, B.E., Simon, J.H., Fischer, U. & Malim, M.H. HIV-1 infection of non-dividing cells: evidence that the amino-terminal basic region of the viral matrix protein is important for Gag processing but not for post-entry nuclear import. EMBO J. 16, 4531–4539 (1997).
Iwabu, Y. et al. Differential anti-APOBEC3G activity of HIV-1 Vif proteins derived from different subtypes. J. Biol. Chem. 285, 35350–35358 (2010).
Ross, T.M., Oran, A.E. & Cullen, B.R. Inhibition of HIV-1 progeny virion release by cell-surface CD4 is relieved by expression of the viral Nef protein. Curr. Biol. 9, 613–621 (1999).
Dong, B. et al. An infectious retrovirus susceptible to an IFN antiviral pathway from human prostate tumors. Proc. Natl. Acad. Sci. USA 104, 1655–1660 (2007).
Shalem, O. et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84–87 (2014).
Nomaguchi, M., Doi, N. & Adachi, A. Virological characterization of HIV-2 vpx gene mutants in various cell systems. Microbes Infect. 16, 695–701 (2014).
Nakai-Murakami, C. et al. HIV-1 Vpr induces ATM-dependent cellular signal with enhanced homologous recombination. Oncogene 26, 477–486 (2007).
Otake, K. et al. The carboxyl-terminal region of HIV-1 Nef protein is a cell surface domain that can interact with CD4+ T cells. J. Immunol. 153, 5826–5837 (1994).
Maldarelli, F., Chen, M.Y., Willey, R.L. & Strebel, K. Human immunodeficiency virus type 1 Vpu protein is an oligomeric type I integral membrane protein. J. Virol. 67, 5056–5061 (1993).
Koyama, T., Sun, B., Tokunaga, K., Tatsumi, M. & Ishizaka, Y. DNA damage enhances integration of HIV-1 into macrophages by overcoming integrase inhibition. Retrovirology 10, 21 (2013).
Tsunetsugu-Yokota, Y. et al. Monocyte-derived cultured dendritic cells are susceptible to human immunodeficiency virus infection and transmit virus to resting T cells in the process of nominal antigen presentation. J. Virol. 69, 4544–4547 (1995).
Perez-Caballero, D. et al. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell 139, 499–511 (2009).
Acknowledgements
We are grateful to F. Barre-Sinoussi for critical reading of the manuscript. We thank D. Trono (Ecole Polytechnique Fédérale de Lausanne, Switzerland, for pWPI; pLVTHM; and pMDLg/pRRE), M.D. Miller (Merck, USA, for pMM310), K. Tomonaga (Kyoto University, Japan, for pBDV-G), Y. Koyanagi (Kyoto University, Japan, for pJRFL); R.H. Silverman and B. Dong (US National Institutes of Health (NIH) AIDS Research and Reference Program, for VP62/pcDNA3.1) F. Zhang (Massachusetts Institute of Technology, USA, for lentiCRISPR-v2), A. Adachi (the University of Tokushima, Japan, for pGL-AN-ΔEnv+Luc), Y. Ishizaka (National Center for Global Health and Medicine, Japan, for 8D1), K. Ikuta (Osaka University, Japan, for 4H4), F. Maldarelli and K. Strebel (US NIH AIDS Research and Reference Program, for anti-Vpu rabbit serum), and T. Sata (Toyama Institute of Health, Japan, for Nu24). This work was supported by grants from the Ministry of Health, Labor and Welfare of Japan (Research on HIV/AIDS project no. H24-005, H24-008 and H25-010 to K.T.), the Japan Society for the Promotion of Science (KAKENHI 26460086 to H.F.), the Japan Agency for Medical Research and Development, AMED (Research Program on HIV/AIDS) (to K.T.). T.T. is a recipient of a Research Resident Fellowship from the Japan Foundation for AIDS Prevention. Y.Z. is a recipient of the Otsuka Toshimi Foundation Scholarship.
Author information
Authors and Affiliations
Contributions
K.T. and H.F. designed experiments and interpreted the data. T.T., Y.Z., T.K., Y.T.-Y., H.F. and K.T. performed experiments and analyzed the data. T.T., Y.Z., Y.T.-Y., S.Y., H.F. and K.T. discussed the data. Y.T.-Y. and M.T. provided reagents. K.T. conceived the study, supervised the work and wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–15 (PDF 1944 kb)
Rights and permissions
About this article
Cite this article
Tada, T., Zhang, Y., Koyama, T. et al. MARCH8 inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins. Nat Med 21, 1502–1507 (2015). https://doi.org/10.1038/nm.3956
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3956
This article is cited by
-
The E3 ligase RNF5 restricts SARS-CoV-2 replication by targeting its envelope protein for degradation
Signal Transduction and Targeted Therapy (2023)
-
When MARCH family proteins meet viral infections
Virology Journal (2021)
-
Hyaluronic acid is a negative regulator of mucosal fibroblast-mediated enhancement of HIV infection
Mucosal Immunology (2021)
-
MARCH8 inhibits influenza A virus infection by targeting viral M2 protein for ubiquitination-dependent degradation in lysosomes
Nature Communications (2021)
-
CRISPR-mediated activation of endogenous BST-2/tetherin expression inhibits wild-type HIV-1 production
Scientific Reports (2019)