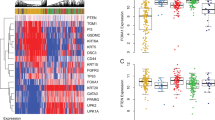
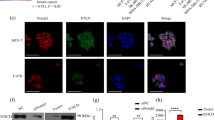
Abstract
The Notch signaling pathway controls cell fates through interactions between neighboring cells by positively or negatively affecting the processes of proliferation, differentiation and apoptosis in a context-dependent manner1. This pathway has been implicated in human cancer as both an oncogene and a tumor suppressor2. Here we report new inactivating mutations in Notch pathway components in over 40% of human bladder cancers examined. Bladder cancer is the fourth most commonly diagnosed malignancy in the male population of the United States3. Thus far, driver mutations in fibroblast growth factor receptor 3 (FGFR3) and, less commonly, in RAS proteins have been identified4,5. We show that Notch activation in bladder cancer cells suppresses proliferation both in vitro and in vivo by directly upregulating dual-specificity phosphatases (DUSPs), thus reducing the phosphorylation of ERK1 and ERK2 (ERK1/2). In mouse models, genetic inactivation of Notch signaling leads to Erk1/2 phosphorylation, resulting in tumorigenesis in the urinary tract. Collectively our findings show that loss of Notch activity is a driving event in urothelial cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Primary accessions
Gene Expression Omnibus
Referenced accessions
NCBI Reference Sequence
References
Artavanis-Tsakonas, S., Rand, M.D. & Lake, R.J. Notch signaling: cell fate control and signal integration in development. Science 284, 770–776 (1999).
Lobry, C., Oh, P. & Aifantis, I. Oncogenic and tumor suppressor functions of Notch in cancer: it's NOTCH what you think. J. Exp. Med. 208, 1931–1935 (2011).
Jemal, A., Siegel, R., Xu, J. & Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 60, 277–300 (2010).
Cordon-Cardo, C. Molecular alterations associated with bladder cancer initiation and progression. Scand. J. Urol. Nephrol. Suppl. 154–165 (2008).
Knowles, M.A. Molecular pathogenesis of bladder cancer. Int. J. Clin. Oncol. 13, 287–297 (2008).
Nicolas, M. et al. Notch1 functions as a tumor suppressor in mouse skin. Nat. Genet. 33, 416–421 (2003).
Lefort, K. et al. Notch1 is a p53 target gene involved in human keratinocyte tumor suppression through negative regulation of ROCK1/2 and MRCKα kinases. Genes Dev. 21, 562–577 (2007).
Klinakis, A. et al. A novel tumour-suppressor function for the Notch pathway in myeloid leukaemia. Nature 473, 230–233 (2011).
Wang, N.J. et al. Loss-of-function mutations in Notch receptors in cutaneous and lung squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 108, 17761–17766 (2011).
Agrawal, N. et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science 333, 1154–1157 (2011).
Stransky, N. et al. The mutational landscape of head and neck squamous cell carcinoma. Science 333, 1157–1160 (2011).
Jerez, A. et al. Topography, clinical, and genomic correlates of 5q myeloid malignancies revisited. J. Clin. Oncol. 30, 1343–1349 (2012).
Kimura, F. et al. Destabilization of chromosome 9 in transitional cell carcinoma of the urinary bladder. Br. J. Cancer 85, 1887–1893 (2001).
Adzhubei, I.A. et al. A method and server for predicting damaging missense mutations. Nat. Methods 7, 248–249 (2010).
Funahashi, Y. et al. A notch1 ectodomain construct inhibits endothelial notch signaling, tumor growth, and angiogenesis. Cancer Res. 68, 4727–4735 (2008).
Cancer Genome Atlas Research. N. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 507, 315–322 (2014).
Guo, G. et al. Whole-genome and whole-exome sequencing of bladder cancer identifies frequent alterations in genes involved in sister chromatid cohesion and segregation. Nat. Genet. 45, 1459–1463 (2013).
Iyer, G. et al. Prevalence and co-occurrence of actionable genomic alterations in high-grade bladder cancer. J. Clin. Oncol. 31, 3133–3140 (2013).
Cazier, J.B. et al. Whole-genome sequencing of bladder cancers reveals somatic CDKN1A mutations and clinicopathological associations with mutation burden. Nat. Commun. 5, 3756 (2014).
Cerami, E. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 (2012).
Weng, A.P. et al. Growth suppression of pre-T acute lymphoblastic leukemia cells by inhibition of notch signaling. Mol. Cell. Biol. 23, 655–664 (2003).
Maraver, A. et al. Therapeutic effect of γ-secretase inhibition in KrasG12V-driven non-small cell lung carcinoma by derepression of DUSP1 and inhibition of ERK. Cancer Cell 22, 222–234 (2012).
Yu, H.M., Liu, B., Chiu, S.Y., Costantini, F. & Hsu, W. Development of a unique system for spatiotemporal and lineage-specific gene expression in mice. Proc. Natl. Acad. Sci. USA 102, 8615–8620 (2005).
Perl, A.K., Wert, S.E., Nagy, A., Lobe, C.G. & Whitsett, J.A. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc. Natl. Acad. Sci. USA 99, 10482–10487 (2002).
Karni-Schmidt, O. et al. Distinct expression profiles of p63 variants during urothelial development and bladder cancer progression. Am. J. Pathol. 178, 1350–1360 (2011).
Shin, K. et al. Cellular origin of bladder neoplasia and tissue dynamics of its progression to invasive carcinoma. Nat. Cell Biol. 16, 469–478 (2014).
Choi, W. et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 25, 152–165 (2014).
Ho, P.L., Kurtova, A. & Chan, K.S. Normal and neoplastic urothelial stem cells: getting to the root of the problem. Nat. Rev. Urol. 9, 583–594 (2012).
Buonamici, S. et al. CCR7 signalling as an essential regulator of CNS infiltration in T-cell leukaemia. Nature 459, 1000–1004 (2009).
Dotto, G.P. Crosstalk of Notch with p53 and p63 in cancer growth control. Nat. Rev. Cancer 9, 587–595 (2009).
Han, H. et al. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int. Immunol. 14, 637–645 (2002).
Barker, N. et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457, 608–611 (2009).
Ahmad, I. et al. K-Ras and β-catenin mutations cooperate with Fgfr3 mutations in mice to promote tumorigenesis in the skin and lung, but not in the bladder. Dis. Model. Mech. 4, 548–555 (2011).
Zhou, H. et al. Urothelial tumor initiation requires deregulation of multiple signaling pathways: implications in target-based therapies. Carcinogenesis 33, 770–780 (2012).
Livak, K.J. & Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC(T) method. Methods 25, 402–408 (2001).
Acknowledgements
We thank J. Kitajewski (Columbia University), P. Dotto (University of Lausanne), M. Post (Maastricht University), D. Stravopodis (University f Athens) and K. Zoi (Biomedical Research Foundation Academy of Athens) for donating reagents, S. Artavanis-Tsakonas (Harvard Medical School) for donating mice, Z. Kanaki for pronuclear injections for the generation of the UpkII-Cre-eGFP line, M. Roubelaki for help with the cell cycle analysis and A. Efstratiadis for critically reading the manuscript. This work was supported by a Marie Curie Reintegration grant (224821), a Greek General Secretariat for Research and Technology 'Excellence' grant (UTN_1466) and a Fondation Santé Grant in Biomedical Sciences to A.K.
Author information
Authors and Affiliations
Contributions
A.K. and T.R. conceived the study and designed all experiments. T.R., P.V. and M.A. performed all experiments. A.P. analyzed expression array data. C.V. performed the mouse histology analysis. A.S. and K.S. provided the library of human tumor samples. A.K. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 and Supplementary Tables 1–10 (PDF 4201 kb)
Rights and permissions
About this article
Cite this article
Rampias, T., Vgenopoulou, P., Avgeris, M. et al. A new tumor suppressor role for the Notch pathway in bladder cancer. Nat Med 20, 1199–1205 (2014). https://doi.org/10.1038/nm.3678
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3678
This article is cited by
-
The urothelial gene regulatory network: understanding biology to improve bladder cancer management
Oncogene (2024)
-
Increased retinoic acid signaling decreases lung metastasis in salivary adenoid cystic carcinoma by inhibiting the noncanonical Notch1 pathway
Experimental & Molecular Medicine (2023)
-
Laminin-integrin a6b4 interaction activates notch signaling to facilitate bladder cancer development
BMC Cancer (2022)
-
Notch signaling pathway: architecture, disease, and therapeutics
Signal Transduction and Targeted Therapy (2022)
-
Tackling tumor microenvironment through epigenetic tools to improve cancer immunotherapy
Clinical Epigenetics (2021)