Abstract
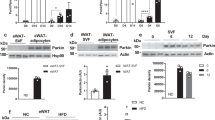
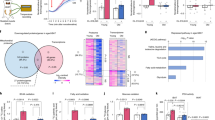
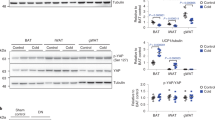
Despite growing interest and a recent surge in papers, the role of autophagy in glucose and lipid metabolism is unclear. We produced mice with skeletal muscle–specific deletion of Atg7 (encoding autophagy-related 7). Unexpectedly, these mice showed decreased fat mass and were protected from diet-induced obesity and insulin resistance; this phenotype was accompanied by increased fatty acid oxidation and browning of white adipose tissue (WAT) owing to induction of fibroblast growth factor 21 (Fgf21). Mitochondrial dysfunction induced by autophagy deficiency increased Fgf21 expression through induction of Atf4, a master regulator of the integrated stress response. Mitochondrial respiratory chain inhibitors also induced Fgf21 in an Atf4-dependent manner. We also observed induction of Fgf21, resistance to diet-induced obesity and amelioration of insulin resistance in mice with autophagy deficiency in the liver, another insulin target tissue. These findings suggest that autophagy deficiency and subsequent mitochondrial dysfunction promote Fgf21 expression, a hormone we consequently term a 'mitokine', and together these processes promote protection from diet-induced obesity and insulin resistance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Levine, B. & Kroemer, G. Autophagy in the pathogenesis of disease. Cell 132, 27–42 (2008).
Ebato, C. et al. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab. 8, 325–332 (2008).
Jung, H.S. et al. Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab. 8, 318–324 (2008).
Singh, R. et al. Autophagy regulates lipid metabolism. Nature 458, 1131–1135 (2009).
Singh, R. et al. Autophagy regulates adipose mass and differentiation in mice. J. Clin. Invest. 119, 3329–3339 (2009).
Zhang, Y. et al. Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis. Proc. Natl. Acad. Sci. USA 106, 19860–19865 (2009).
Yang, L., Li, P., Fu, S., Calay, E.S. & Hotamisligil, G.S. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 11, 467–478 (2010).
Wu, J.J. et al. Mitochondrial dysfunction and oxidative stress mediate the physiological impairment induced by the disruption of autophagy. Aging (Albany NY) 1, 425–437 (2009).
Wen, H. et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 12, 408–415 (2011).
Komatsu, M. et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J. Cell Biol. 169, 425–434 (2005).
Masiero, E. et al. Autophagy is required to maintain muscle mass. Cell Metab. 10, 507–515 (2009).
Mortensen, M. et al. Loss of autophagy in erythroid cells leads to defective removal of mitochondria and severe anemia in vivo. Proc. Natl. Acad. Sci. USA 107, 832–837 (2010).
Petersen, K.F. et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300, 1140–1142 (2003).
Mootha, V.K. et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34, 267–273 (2003).
Patti, M.E. et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc. Natl. Acad. Sci. USA 100, 8466–8471 (2003).
Boushel, R. et al. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia 50, 790–796 (2007).
Hancock, C.R. et al. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc. Natl. Acad. Sci. USA 105, 7815–7820 (2008).
Pospisilik, J.A. et al. Targeted deletion of AIF decreases mitochondrial oxidative phosphorylation and protects from obesity and diabetes. Cell 131, 476–491 (2007).
Wredenberg, A. et al. Respiratory chain dysfunction in skeletal muscle does not cause insulin resistance. Biochem. Biophys. Res. Commun. 350, 202–207 (2006).
Baron, A.D., Brechtel, G., Wallace, P. & Edelman, S.V. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am. J. Physiol. 255, E769–E774 (1988).
Tschöp, M. et al. A guide to analysis of mouse energy metabolism. Nat. Methods 9, 57–63 (2001).
Puigserver, P. et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839 (1998).
Danforth, E., Jr. & Burger, A. The role of thyroid hormones in the control of energy expenditure. Clin. Endocrinol. Metab. 13, 581–595 (1984).
Halaas, J.L. et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269, 543–546 (1995).
Yamauchi, T. et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 7, 941–946 (2001).
Handschin, C. et al. Abnormal glucose homeostasis in skeletal muscle-specific PGC-1α knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. J. Clin. Invest. 117, 3463–3474 (2007).
Izumiya, Y. et al. FGF21 is an Akt-regulated myokine. FEBS Lett. 582, 3805–3810 (2008).
Kharitonenkov, A. et al. FGF-21 as a novel metabolic regulator. J. Clin. Invest. 115, 1627–1635 (2005).
Xu, J. et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 58, 250–259 (2009).
Coskun, T. et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149, 6018–6027 (2008).
Potthoff, M.J. et al. FGF21 induces PGC-1α and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc. Natl. Acad. Sci. USA 106, 10853–10858 (2009).
Inagaki, T. et al. Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab. 5, 415–425 (2007).
Fisher, F.M. et al. Integrated regulation of hepatic metabolism by fibroblast growth factor 21 (FGF21) in vivo. Endocrinology 152, 2996–3004 (2011).
Chen, W. et al. Growth hormone induces hepatic production of fibroblast growth factor 21 through a mechanism dependent on lipolysis in adipocytes. J. Biol. Chem. 286, 34559–34566 (2011).
Harding, H.P. et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 11, 619–633 (2003).
Dey, S. et al. Both transcriptional regulation and translational control of ATF4 are central to the integrated stress response. J. Biol. Chem. 285, 33165–33174 (2010).
Komatsu, M. et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 12, 213–223 (2010).
Miyamoto, N. et al. Transcriptional regulation of activating transcription factor 4 under oxidative stress in retinal pigment epithelial ARPE-19/HPV-16 cells. Invest. Ophthalmol. Vis. Sci. 52, 1226–1234 (2011).
Silva, J.M., Wong, A., Carelli, V. & Cortopassi, G.A. Inhibition of mitochondrial function induces an integrated stress response in oligodendroglia. Neurobiol. Dis. 34, 357–365 (2009).
Bouman, L. et al. Parkin is transcriptionally regulated by ATF4: evidence for an interconnection between mitochondrial stress and ER stress. Cell Death Differ. 18, 769–782 (2011).
Chen, H. et al. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 141, 280–289 (2010).
Koga, H., Kaushik, S. & Cuervo, A.M. Altered lipid content inhibits autophagic vesicular fusion. FASEB J. 24, 3052–3065 (2010).
Tyynismaa, H. et al. Mitochondrial myopathy induces a starvation-like response. Hum. Mol. Genet. 19, 3948–3958 (2010).
Suomalainen, A. et al. FGF-21 as a biomarker for muscle-manifesting mitochondrial respiratory chain deficiencies: a diagnostic study. Lancet Neurol. 10, 806–818 (2011).
Durieux, J., Wolff, S. & Dillin, A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell 144, 79–91 (2011).
Kusminski, C.M. et al. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat. Med. 18, 1539–1549 (2012).
Ibrahimi, A. et al. Muscle-specific overexpression of FAT/CD36 enhances fatty acid oxidation by contracting muscle, reduces plasma triglycerides and fatty acids, and increases plasma glucose and insulin. J. Biol. Chem. 274, 26761–26766 (1999).
Storch, J. & Thumser, A.E. Tissue-specific functions in the fatty acid-binding protein family. J. Biol. Chem. 285, 32679–32683 (2010).
Fisher, F.M. et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 26, 271–281 (2012).
Silva, J.P. et al. Impaired insulin secretion and beta-cell loss in tissue-specific knockout mice with mitochondrial diabetes. Nat. Genet. 26, 336–340 (2000).
He, C. et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature 481, 511–515 (2012).
Shibata, M. et al. The MAP1–LC3 conjugation system is involved in lipid droplet formation. Biochem. Biophys. Res. Commun. 382, 419–423 (2009).
Donnelly, K.L. et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 115, 1343–1351 (2005).
Lin, J. et al. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1β coactivation of SREBP. Cell 120, 261–73 (2005).
Savage, D.B. et al. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J. Clin. Invest. 116, 817–824 (2006).
Gutiérrez-Juárez, R. et al. Critical role of stearoyl-CoA desaturase-1 (SCD1) in the onset of diet-induced hepatic insulin resistance. J. Clin. Invest. 116, 1686–1695 (2006).
Choi, C.S. et al. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J. Biol. Chem. 282, 22678–22688 (2007).
Bothe, G.W., Haspel, J.A., Smith, C.L., Wiener, H.H. & Burden, S.J. Selective expression of Cre recombinase in skeletal muscle fibers. Genesis 26, 165–166 (2000).
Hotta, Y. et al. Fibroblast growth factor 21 regulates lipolysis in white adipose tissue but is not required for ketogenesis and triglyceride clearance in liver. Endocrinology 150, 4625–4633 (2009).
Seligman, A.M., Karnovsky, M.J., Wasserkrug, H.L. & Hanker, J.S. Nondroplet ultrastructural demonstration of cytochrome oxidase activity with a polymerizing osmiophilic reagent, diaminobenzidine (DAB). J. Cell Biol. 38, 1–14 (1968).
Acknowledgements
We thank S.R. Farmer (Boston University) for pGL3B-Fgf21 (−1370/+129), D. Ron (University of Cambridge) for Atf4-null MEFs, R.J. Kaufman (University of Michigan Medical Center) for Eif2aS/S and Eif2aA/A MEFs, S.J. Burden (New York University) for Mlc1f-Cre mice, N. Mizushima (Tokyo Medical and Dental University) for Tet-off Atg5-null MEFs, and H.W. Virgin for insightful comments on the manuscript. This study was supported by the Global Research Laboratory Grant of the National Research Foundation of Korea (K21004000003-10A0500-00310 to M.-S.L. and M. Komatsu) and the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Korea (A080967 to M.-S.L. and A102060 to C.S.C). M.-S.L. received the Bio R&D Program (2008-04090) and the Bio & Medical Technology Development Program grant from the Ministry of Education, Science & Technology, Korea (20110019335).
Author information
Authors and Affiliations
Contributions
K.H.K., C.S.C. and M.-S.L. designed the study, analyzed data and wrote the manuscript. K.H.K. conducted all experiments except the portions indicated below, assisted by S.H.K., J.M.C., D.H.K. and K.Y.H. Y.T.J. analyzed the metabolic profiling of Atg7Δhep mice. H.O., Y.-N.K. and S.S.K. performed measurements of body composition, indirect calorimetry, the hyperinsulinemic-euglycemic clamp study and the fatty acid oxidation experiments. H.K.K., T.K. and J.H. measured the mitochondrial oxygen consumption. H.L.K. and J.K. performed the electron microscopy. S.H.B., M. Komatsu, H.C., D.C.C., M. Konishi and N.I. provided reagents, tissues, cells or mice and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Figures 1–11 and Supplementary Methods (PDF 2560 kb)
Rights and permissions
About this article
Cite this article
Kim, K., Jeong, Y., Oh, H. et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat Med 19, 83–92 (2013). https://doi.org/10.1038/nm.3014
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3014
This article is cited by
-
Mammalian integrated stress responses in stressed organelles and their functions
Acta Pharmacologica Sinica (2024)
-
ATF4 expression in thermogenic adipocytes is required for cold-induced thermogenesis in mice via FGF21-independent mechanisms
Scientific Reports (2024)
-
Causal relationship between insulin resistance and sarcopenia
Diabetology & Metabolic Syndrome (2023)
-
Development of muscle weakness in a mouse model of critical illness: does fibroblast growth factor 21 play a role?
Skeletal Muscle (2023)
-
Crosstalk between autophagy and insulin resistance: evidence from different tissues
European Journal of Medical Research (2023)