Abstract
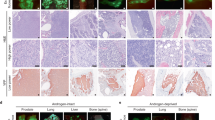
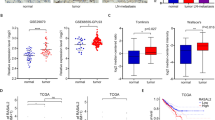
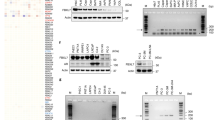
Metastasis is responsible for the majority of prostate cancer–related deaths; however, little is known about the molecular mechanisms that underlie this process. Here we identify an oncogene–tumor suppressor cascade that promotes prostate cancer growth and metastasis by coordinately activating the small GTPase Ras and nuclear factor-κB (NF-κB). Specifically, we show that loss of the Ras GTPase-activating protein (RasGAP) gene DAB2IP induces metastatic prostate cancer in an orthotopic mouse tumor model. Notably, DAB2IP functions as a signaling scaffold that coordinately regulates Ras and NF-κB through distinct domains to promote tumor growth and metastasis, respectively. DAB2IP is suppressed in human prostate cancer, where its expression inversely correlates with tumor grade and predicts prognosis. Moreover, we report that epigenetic silencing of DAB2IP is a key mechanism by which the polycomb-group protein histone-lysine N-methyltransferase EZH2 activates Ras and NF-κB and triggers metastasis. These studies define the mechanism by which two major pathways can be simultaneously activated in metastatic prostate cancer and establish EZH2 as a driver of metastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
21 February 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41591-024-02866-2
References
Nelson, W.G., De Marzo, A.M. & Isaacs, W.B. Prostate cancer. N. Engl. J. Med. 349, 366–381 (2003).
Jemal, A. et al. Cancer statistics, 2008. CA Cancer J. Clin. 58, 71–96 (2008).
Jeong, J.H. et al. BRAF activation initiates but does not maintain invasive prostate adenocarcinoma. PLoS One 3, e3949 (2008).
Shen, M.M. & Abate-Shen, C. Pten inactivation and the emergence of androgen-independent prostate cancer. Cancer Res. 67, 6535–6538 (2007).
Gioeli, D., Mandell, J.W., Petroni, G.R., Frierson, H.F. Jr. & Weber, M.J. Activation of mitogen-activated protein kinase associated with prostate cancer progression. Cancer Res. 59, 279–284 (1999).
Malik, S.N. et al. Immunohistochemical demonstration of phospho-Akt in high Gleason grade prostate cancer. Clin. Cancer Res. 8, 1168–1171 (2002).
Carter, B.S., Epstein, J.I. & Isaacs, W.B. ras gene mutations in human prostate cancer. Cancer Res. 50, 6830–6832 (1990).
Gumerlock, P.H., Poonamallee, U.R., Meyers, F.J. & deVere White, R.W. Activated ras alleles in human carcinoma of the prostate are rare. Cancer Res. 51, 1632–1637 (1991).
Cho, N.Y. et al. BRAF and KRAS mutations in prostatic adenocarcinoma. Int. J. Cancer 119, 1858–1862 (2006).
Bernards, A. & Settleman, J. GEFs in growth factor signaling. Growth Factors 25, 355–361 (2007).
Bernards, A. & Settleman, J. GAPs in growth factor signaling. Growth Factors 23, 143–149 (2005).
Riccardi, V.M. Neurofibromatosis: Phenotype, Natural History and Pathogenesis. Ch. 3 (The Johns Hopkins University Press, Baltimore, 1992).
Ding, L. et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature 455, 1069–1075 (2008).
Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455, 1061–1068 (2008).
McGillicuddy, L.T. et al. Proteasomal and genetic inactivation of the NF1 tumor suppressor in gliomagenesis. Cancer Cell 16, 44–54 (2009).
Johannessen, C.M. et al. The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc. Natl. Acad. Sci. USA 102, 8573–8578 (2005).
von Bergh, A.R. et al. Identification of a novel RAS GTPase–activating protein (RASGAP) gene at 9q34 as an MLL fusion partner in a patient with de novo acute myeloid leukemia. Genes Chromosom. Cancer 39, 324–334 (2004).
Dote, H. et al. Aberrant promoter methylation in human DAB2 interactive protein (hDAB2IP) gene in breast cancer. Clin. Cancer Res. 10, 2082–2089 (2004).
Dote, H. et al. Aberrant promoter methylation in human DAB2 interactive protein (hDAB2IP) gene in gastrointestinal tumour. Br. J. Cancer 92, 1117–1125 (2005).
Yano, M. et al. Aberrant promoter methylation of human DAB2 interactive protein (hDAB2IP) gene in lung cancers. Int. J. Cancer 113, 59–66 (2005).
Chen, H., Tu, S.W. & Hsieh, J.T. Down-regulation of human DAB2IP gene expression mediated by polycomb Ezh2 complex and histone deacetylase in prostate cancer. J. Biol. Chem. 280, 22437–22444 (2005).
Ke, X.S. et al. Genome-wide profiling of histone h3 lysine 4 and lysine 27 trimethylation reveals an epigenetic signature in prostate carcinogenesis. PLoS One 4, e4687 (2009).
Berger, R. et al. Androgen-induced differentiation and tumorigenicity of human prostate epithelial cells. Cancer Res. 64, 8867–8875 (2004).
Arai, Y. et al. Radical prostatectomy for clinically localized prostate cancer: local tumor extension and prognosis. Int. J. Urol. 3, 373–378 (1996).
Sørensen, H.T., Mellemkjaer, L., Olsen, J.H. & Baron, J.A. Prognosis of cancers associated with venous thromboembolism. N. Engl. J. Med. 343, 1846–1850 (2000).
Scheel, C., Onder, T., Karnoub, A. & Weinberg, R.A. Adaptation versus selection: the origins of metastatic behavior. Cancer Res. 67, 11476–11479, discussion 11479–11480 (2007).
Thiery, J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2, 442–454 (2002).
Weinberg, R.A. The Biology of Cancer. (Garland Science, New York, 2007).
Yang, J. et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117, 927–939 (2004).
Joyce, J.A. & Pollard, J.W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer 9, 239–252 (2009).
Zhang, H. et al. AIP1/DAB2IP, a novel member of the Ras-GAP family, transduces TRAF2-induced ASK1-JNK activation. J. Biol. Chem. 279, 44955–44965 (2004).
Huber, M.A., Beug, H. & Wirth, T. Epithelial-mesenchymal transition: NF-κB takes center stage. Cell Cycle 3, 1477–1480 (2004).
Naugler, W.E. & Karin, M. NF-κB and cancer-identifying targets and mechanisms. Curr. Opin. Genet. Dev. 18, 19–26 (2008).
Hayden, M.S. & Ghosh, S. Shared principles in NF-κB signaling. Cell 132, 344–362 (2008).
Mayo, M.W. et al. Requirement of NF-κB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science 278, 1812–1815 (1997).
Simon, J.A. & Lange, C.A. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat. Res. 647, 21–29 (2008).
Varambally, S. et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 419, 624–629 (2002).
Yu, J. et al. A polycomb repression signature in metastatic prostate cancer predicts cancer outcome. Cancer Res. 67, 10657–10663 (2007).
Kondo, Y. et al. Gene silencing in cancer by histone H3 lysine 27 trimethylation independent of promoter DNA methylation. Nat. Genet. 40, 741–750 (2008).
Vanaja, D.K., Cheville, J.C., Iturria, S.J. & Young, C.Y. Transcriptional silencing of zinc finger protein 185 identified by expression profiling is associated with prostate cancer progression. Cancer Res. 63, 3877–3882 (2003).
Lapointe, J. et al. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc. Natl. Acad. Sci. USA 101, 811–816 (2004).
Tomlins, S.A., Rubin, M.A. & Chinnaiyan, A.M. Integrative biology of prostate cancer progression. Annu. Rev. Pathol. 1, 243–271 (2006).
Luo, J.H. et al. Gene expression analysis of prostate cancers. Mol. Carcinog. 33, 25–35 (2002).
Daskivich, T.J. & Oh, W.K. Recent progress in hormonal therapy for advanced prostate cancer. Curr. Opin. Urol. 16, 173–178 (2006).
Abate-Shen, C. & Shen, M.M. Mouse models of prostate carcinogenesis. Trends Genet. 18, S1–S5 (2002).
Wang, S. et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell 4, 209–221 (2003).
Henry, D.O. et al. Ral GTPases contribute to regulation of cyclin D1 through activation of NF-kappaB. Mol. Cell. Biol. 20, 8084–8092 (2000).
Barbie, D.A. et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature 462, 108–112 (2009).
Sellers, W.R. & Loda, M. The EZH2 polycomb transcriptional repressor—a marker or mover of metastatic prostate cancer? Cancer Cell 2, 349–350 (2002).
Chen, H., Toyooka, S., Gazdar, A.F. & Hsieh, J.T. Epigenetic regulation of a novel tumor suppressor gene (hDAB2IP) in prostate cancer cell lines. J. Biol. Chem. 278, 3121–3130 (2003).
Acknowledgements
The PMMP-luc-neo retroviral luciferase construct was generously provided by A.L. Kung and M. Chheda (Dana-Farber Cancer Institute (DFCI)). pRL-TK was a gift from J. Boehm (Broad Institute). We thank D. Barbie (DFCI), J. Boehm for NF-κB target primers and R. Chen (DFCI) for IκBαSR and G. Evan for helpful discussions. This grant was supported by in part by the US Department of Defense (PC074048) and the Ludwig Center at Dana-Farber/Harvard Cancer Center.
Author information
Authors and Affiliations
Contributions
J.M., A.Z., S.K.M., E.E.R., I.G. and D.E.S. performed in vitro and in vivo experiments. G.F., R.T.B. and M.L. analyzed and interpreted the immunohistochemistry and histology experiments. G.F. performed analysis on tissue microarrays. T.D.R. performed statistical analysis. L.E.M. and R.B. performed Sequenome analysis. S.R., W.C.H. and M.L. provided scientific advice and helpful comments on manuscript. J.M. and K.C. conceptualized experiments, prepared figures and drafted the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1–10 and Supplementary Table 1 (PDF 971 kb)
Rights and permissions
About this article
Cite this article
Min, J., Zaslavsky, A., Fedele, G. et al. An oncogene–tumor suppressor cascade drives metastatic prostate cancer by coordinately activating Ras and nuclear factor-κB. Nat Med 16, 286–294 (2010). https://doi.org/10.1038/nm.2100
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2100
This article is cited by
-
Disable 2, A Versatile Tissue Matrix Multifunctional Scaffold Protein with Multifaceted Signaling: Unveiling Role in Breast Cancer for Therapeutic Revolution
Cell Biochemistry and Biophysics (2024)
-
Disabled-2, a versatile tissue matrix multifunctional scaffold protein with multifaceted signaling: Unveiling its potential in the cancer battle
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
DAB2IP stabilizes p27Kip1 via suppressing PI3K/AKT signaling in clear cell renal cell carcinoma
Functional & Integrative Genomics (2023)
-
Epigenetic markers and therapeutic targets for metastasis
Cancer and Metastasis Reviews (2023)
-
Neuronal Histone Methyltransferase EZH2 Regulates Neuronal Morphogenesis, Synaptic Plasticity, and Cognitive Behavior in Mice
Neuroscience Bulletin (2023)