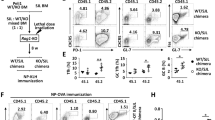
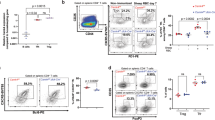
Abstract
Aberrant population expansion of follicular helper T cells (TFH cells) occurs in patients with lupus. An unanswered question is whether an altered repertoire of T cell antigen receptors (TCRs) is associated with such expansion. Here we found that the transcription factor Blimp-1 (encoded by Prdm1) repressed expression of the gene encoding cathepsin S (Ctss), a cysteine protease that cleaves invariant chains and produces antigenic peptides for loading onto major histocompatibility complex (MHC) class II molecules. The increased CTSS expression in dendritic cells (DCs) from female mice with dendritic cell–specific conditional knockout of Prdm1 (CKO mice) altered the presentation of antigen to CD4+ T cells. Analysis of complementarity-determining region 3 (CDR3) regions containing the β-chain variable region (Vβ) demonstrated a more diverse repertoire of TFH cells from female CKO mice than of those from wild-type mice. In vivo treatment of CKO mice with a CTSS inhibitor abolished the lupus-related phenotype and reduced the diversity of the TFH cell TCR repertoire. Thus, Blimp-1 deficiency in DCs led to loss of appropriate regulation of Ctss expression in female mice and thereby modulated antigen presentation and the TFH cell repertoire to contribute to autoimmunity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Pavli, P., Hume, D.A., Van De Pol, E. & Doe, W.F. Dendritic cells, the major antigen-presenting cells of the human colonic lamina propria. Immunology 78, 132–141 (1993).
Guilliams, M. et al. Skin-draining lymph nodes contain dermis-derived CD103− dendritic cells that constitutively produce retinoic acid and induce Foxp3+ regulatory T cells. Blood 115, 1958–1968 (2010).
Bousso, P. T-cell activation by dendritic cells in the lymph node: lessons from the movies. Nat. Rev. Immunol. 8, 675–684 (2008).
Zhu, J. & Paul, W.E. CD4 T cells: fates, functions, and faults. Blood 112, 1557–1569 (2008).
Rogers, P.R. & Croft, M. Peptide dose, affinity, and time of differentiation can contribute to the Th1/Th2 cytokine balance. J. Immunol. 163, 1205–1213 (1999).
Rogers, P.R., Dubey, C. & Swain, S.L. Qualitative changes accompany memory T cell generation: faster, more effective responses at lower doses of antigen. J. Immunol. 164, 2338–2346 (2000).
Théry, C. & Amigorena, S. The cell biology of antigen presentation in dendritic cells. Curr. Opin. Immunol. 13, 45–51 (2001).
Villadangos, J.A. et al. Proteases involved in MHC class II antigen presentation. Immunol. Rev. 172, 109–120 (1999).
Shi, G.P. et al. Human cathepsin S: chromosomal localization, gene structure, and tissue distribution. J. Biol. Chem. 269, 11530–11536 (1994).
Hsieh, C.S., deRoos, P., Honey, K., Beers, C. & Rudensky, A.Y. A role for cathepsin L and cathepsin S in peptide generation for MHC class II presentation. J. Immunol. 168, 2618–2625 (2002).
Beers, C. et al. Cathepsin S controls MHC class II-mediated antigen presentation by epithelial cells in vivo. J. Immunol. 174, 1205–1212 (2005).
Stoeckle, C. et al. Cathepsin S dominates autoantigen processing in human thymic dendritic cells. J. Autoimmun. 38, 332–343 (2012).
Han, J.W. et al. Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nat. Genet. 41, 1234–1237 (2009).
Gateva, V. et al. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat. Genet. 41, 1228–1233 (2009).
Kim, S.J., Gregersen, P.K. & Diamond, B. Regulation of dendritic cell activation by microRNA let-7c and BLIMP1. J. Clin. Invest. 123, 823–833 (2013).
Xu, H. et al. Increased frequency of circulating follicular helper T cells in lupus patients is associated with autoantibody production in a CD40L-dependent manner. Cell. Immunol. 295, 46–51 (2015).
Le Coz, C. et al. Circulating TFH subset distribution is strongly affected in lupus patients with an active disease. PLoS One 8, e75319 (2013).
Choi, J.Y. et al. Circulating follicular helper-like T cells in systemic lupus erythematosus: association with disease activity. Arthritis Rheumatol. 67, 988–999 (2015).
Kim, S.J., Zou, Y.R., Goldstein, J., Reizis, B. & Diamond, B. Tolerogenic function of Blimp-1 in dendritic cells. J. Exp. Med. 208, 2193–2199 (2011).
Piskurich, J.F. et al. BLIMP-I mediates extinction of major histocompatibility class II transactivator expression in plasma cells. Nat. Immunol. 1, 526–532 (2000).
Vander Lugt, B. et al. Transcriptional programming of dendritic cells for enhanced MHC class II antigen presentation. Nat. Immunol. 15, 161–167 (2014).
Doody, G.M. et al. An extended set of PRDM1/BLIMP1 target genes links binding motif type to dynamic repression. Nucleic Acids Res. 38, 5336–5350 (2010).
Seillet, C. et al. Estradiol promotes functional responses in inflammatory and steady-state dendritic cells through differential requirement for activation function-1 of estrogen receptor α. J. Immunol. 190, 5459–5470 (2013).
Kitamura, H. et al. IL-6-STAT3 controls intracellular MHC class II αβ dimer level through cathepsin S activity in dendritic cells. Immunity 23, 491–502 (2005).
Becker, S., Groner, B. & Müller, C.W. Three-dimensional structure of the Stat3β homodimer bound to DNA. Nature 394, 145–151 (1998).
Hutchins, A.P., Diez, D. & Miranda-Saavedra, D. The IL-10/STAT3-mediated anti-inflammatory response: recent developments and future challenges. Brief. Funct. Genomics 12, 489–498 (2013).
Pratama, A. & Vinuesa, C.G. Control of TFH cell numbers: why and how? Immunol. Cell Biol. 92, 40–48 (2014).
Walker, B., Lynas, J.F., Meighan, M.A. & Brömme, D. Evaluation of dipeptide α-keto-β-aldehydes as new inhibitors of cathepsin S. Biochem. Biophys. Res. Commun. 275, 401–405 (2000).
Rupanagudi, K.V. et al. Cathepsin S inhibition suppresses systemic lupus erythematosus and lupus nephritis because cathepsin S is essential for MHC class II-mediated CD4 T cell and B cell priming. Ann. Rheum. Dis. 74, 452–463 (2015).
Klein, L., Hinterberger, M., Wirnsberger, G. & Kyewski, B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat. Rev. Immunol. 9, 833–844 (2009).
Deng, Y. & Tsao, B.P. Genetic susceptibility to systemic lupus erythematosus in the genomic era. Nat. Rev. Rheumatol. 6, 683–692 (2010).
Raj, P. et al. Regulatory polymorphisms modulate the expression of HLA class II molecules and promote autoimmunity. eLife 5, e12089 (2016).
Yang, H., Rittner, H., Weyand, C.M. & Goronzy, J.J. Aberrations in the primary T-cell receptor repertoire as a predisposition for synovial inflammation in rheumatoid arthritis. J. Investig. Med. 47, 236–245 (1999).
Yang, H. et al. Hrd1-mediated BLIMP-1 ubiquitination promotes dendritic cell MHCII expression for CD4 T cell priming during inflammation. J. Exp. Med. 211, 2467–2479 (2014).
Honey, K. & Rudensky, A.Y. Lysosomal cysteine proteases regulate antigen presentation. Nat. Rev. Immunol. 3, 472–482 (2003).
Yang, H. et al. Cathepsin S is required for murine autoimmune myasthenia gravis pathogenesis. J. Immunol. 174, 1729–1737 (2005).
Tato, M. et al. Cathepsin S inhibition combines control of systemic and peripheral pathomechanisms of autoimmune tissue injury. Sci. Rep. 7, 2775 (2017).
Yu, D. et al. Roquin represses autoimmunity by limiting inducible T-cell co-stimulator messenger RNA. Nature 450, 299–303 (2007).
Kim, S.J. et al. Increased IL-12 inhibits B cells' differentiation to germinal center cells and promotes differentiation to short-lived plasmablasts. J. Exp. Med. 205, 2437–2448 (2008).
Brocker, T. Survival of mature CD4 T lymphocytes is dependent on major histocompatibility complex class II-expressing dendritic cells. J. Exp. Med. 186, 1223–1232 (1997).
Goldrath, A.W. & Bevan, M.J. Selecting and maintaining a diverse T-cell repertoire. Nature 402, 255–262 (1999).
Rocha, B. & von Boehmer, H. Peripheral selection of the T cell repertoire. Science 251, 1225–1228 (1991).
Ndifon, W. et al. Chromatin conformation governs T-cell receptor Jβ gene segment usage. Proc. Natl. Acad. Sci. USA 109, 15865–15870 (2012).
Acknowledgements
We thank H. Borrero and C. Colon for assistance with the flow cytometry; G. Klein and M. DeFranco RN for recruiting PRDM1-genotyped subjects; and C. Chrysostomou for discussions and assistance in bioinformatics analysis. Supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases, the US National Institutes of Health (R01 AR065209 to S.J.K. and B.D.), the Alliance of Lupus Research (B.D.) and the Defense Threat Reduction Agency (HDTRA 1-12-C-0105 for G.G.).
Author information
Authors and Affiliations
Contributions
S.J.K. designed and performed experiments, analyzed data and interpreted results; S.S. performed the sequencing experiments and analyzed data; S.S.A. and W.H. provided RO5461111, and critically reviewed the manuscript; S.H.J. performed the ChIP experiments and Il6 promoter assay; P.K.G. provided the samples for the human study; G.G. designed the sequencing experiments and interpreted data; B.D. designed the study and analyzed and interpreted results; S.J.K., S.S., G.G. and B.D. participated in the interpretation of the study and wrote the manuscript, and also provided critical review of the paper; and all contributing authors agreed to the submission of this manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
S.S.A. and W.H. are employed by and hold stock in Roche Pharmaceuticals.
Integrated supplementary information
Supplementary Figure 1 Expression of cathepsin-encoding genes in splenic DCs from wild-type or CKO mice.
DCs were purified from the spleens of age matched female wild-type or CKO mice, and RNA was purified for qPCR. Gene specific primers were used for each cathepsin gene and the relative expression of each was calculated by normalization to the level of Hprt. Each dot represents an individual mouse and the bar represents the mean ± SEM (n=5).
Supplementary Figure 2 Increased expression of Il6 by estrogen in BM-DCs.
BM-DCs were differentiated with GM-CSF (200 ng/ml) in hormone-free culture system without or with E2, ICI or E2 and ICI together for 7 days. On day 7, BM-DCs were counted and further cultured with or without LPS (1 μg/ml) for overnight. Total RNA was purified and Il6 transcript was assessed by qPCR. Relative level of Il6 was normalized to housekeeping gene Polr2a. In the box-and-whisker plot, horizontal bars indicate the median, boxes indicate 25th to 75th percentile, and whiskers indicate 10th and 90th percentile (n=7).
Supplementary Figure 4 Gene expression in TFH and non-TFH cells.
Spleens were collected from age-matched female wild-type (open circle) or CKO (closed circle) mice. TFH cells and non-TFH cells were identified as represented in the flow image (left panel), and each population was purified. Total RNA was purified and gene expression was measured by qPCR. Relative level of each gene was normalized to housekeeping gene Polr2a. Each dot represents an individual mouse and the bar represents the mean ± SEM (n=3).
Supplementary Figure 5 Increased IL-21 protein in the supernatant from co-culture of OT-II CD4+ T cells and Blimp-1-deficient DCs.
CD4+ T cells from OT-II mice and splenic DCs (open box is wild-type mice and gray box is CKO mice) were co-cultured in various conditions for 4 days. To measure the secreted cytokines, IL-2 and IL-21, supernatant was collected and cytokines were detected by a multiplex assay. In the box-and-whisker plot, horizontal bars indicate the median, boxes indicate 25th to 75th percentile, and whiskers indicate 10th and 90th percentile (n=6).
Supplementary Figure 6 Cytokine production in the supernatant from day 3 co-cultures of OT-II T cells and DCs.
DCs were purified from the spleens of female wild-type (open box) or CKO (gray box) mice, and CD4+ T cells were purified from female OT-II mice. DCs and T cells were co-cultured for 3 days at a 1:10 ratio (DC:T) with either OVA323-339 peptide (100 ng/ml) or OVA protein (10 μg/ml). Cytokine levels were measured in the supernatant by multiplex assay. In the box-and-whisker plot, horizontal bars indicate the median, boxes indicate 25th to 75th percentile, and whiskers indicate 10th and 90th percentile (n=6).
Supplementary Figure 7 Proliferation and IL-21 production of OT-II T cells by DCs presenting OVA peptide (amino acids 323–339).
OT-II T cells were co-cultured with splenic DCs for 3 days at 1:10 ratio (DC:T cells) in the presence of OVA323-339 peptide. To assess proliferation, T cells were labeled with CFSE (10 mM) before the culture and proliferation was measured by calculation of the division index (left panel). In the box-and-whisker plot, horizontal bars indicate the median, boxes indicate 25th to 75th percentile, and whiskers indicate 10th and 90th percentile (n=3). To assess IL-21 production, culture supernatant was harvested and measured by Multiplex assay (right panel). In the box-and-whisker plot, horizontal bars indicate the median, boxes indicate 25th to 75th percentile, and whiskers indicate 10th and 90th percentile (n=3).
Supplementary Figure 8 Protein level of cytokines, IL-2 and IL-21, measured by intracellular staining of T cells.
OT-II T cells were co-cultured with splenic DCs that had been incubated with HEL (10 μ/ml) or OVA (10 μg/ml). CTSB inhibitor (1 nM) was added during processing of OVA protein antigen. After co-culture for 4 days, T cells were stimulated with PMA (100 ng/ml)/ionomycin (1 μg/ml) for 6 hours. BFA (20 μg/ml) was added during the last 4 hours of stimulation. Cytokine-positive cells were identified by flow cytometry and frequency was calculated and presented in the graph. Each dot represents an individual experiment and the bar represents the mean ± SEM (n=4).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Table 1 (PDF 689 kb)
Rights and permissions
About this article
Cite this article
Kim, S., Schätzle, S., Ahmed, S. et al. Increased cathepsin S in Prdm1−/− dendritic cells alters the TFH cell repertoire and contributes to lupus. Nat Immunol 18, 1016–1024 (2017). https://doi.org/10.1038/ni.3793
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3793
This article is cited by
-
A Contemporary Update on the Diagnosis of Systemic Lupus Erythematosus
Clinical Reviews in Allergy & Immunology (2022)
-
Targeting lysosomes in human disease: from basic research to clinical applications
Signal Transduction and Targeted Therapy (2021)
-
CXCR5-negative natural killer cells ameliorate experimental autoimmune myasthenia gravis by suppressing follicular helper T cells
Journal of Neuroinflammation (2019)
-
Regulation of Cathepsin E gene expression by the transcription factor Kaiso in MRL/lpr mice derived CD4+ T cells
Scientific Reports (2019)
-
Estrogen receptor α in T cells suppresses follicular helper T cell responses and prevents autoimmunity
Experimental & Molecular Medicine (2019)