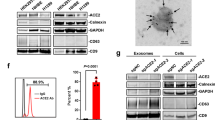
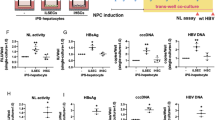
Abstract
The cell-to-cell transmission of viral resistance is a potential mechanism for amplifying the interferon-induced antiviral response. In this study, we report that interferon-α (IFN-α) induced the transfer of resistance to hepatitis B virus (HBV) from nonpermissive liver nonparenchymal cells (LNPCs) to permissive hepatocytes via exosomes. Exosomes from IFN-α-treated LNPCs were rich in molecules with antiviral activity. Moreover, exosomes from LNPCs were internalized by hepatocytes, which mediated the intercellular transfer of antiviral molecules. Finally, we found that exosomes also contributed to the antiviral response of IFN-α to mouse hepatitis virus A59 and adenovirus in mice. Thus, we propose an antiviral mechanism of IFN-α activity that involves the induction and intercellular transfer of antiviral molecules via exosomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
Primary accessions
ArrayExpress
Referenced accessions
NCBI Reference Sequence
References
Borden, E.C. et al. Interferons at age 50: past, current and future impact on biomedicine. Nat. Rev. Drug Discov. 6, 975–990 (2007).
Chevaliez, S. & Pawlotsky, J.M. Interferons and their use in persistent viral infections. Handb. Exp. Pharmacol. 189, 203–241 (2009).
Randall, R.E. & Goodbourn, S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 89, 1–47 (2008).
Blalock, J.E. & Baron, S. Interferon-induced transfer of viral resistance between animal cells. Nature 269, 422–425 (1977).
Théry, C., Ostrowski, M. & Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 9, 581–593 (2009).
Mittelbrunn, M. & Sanchez-Madrid, F. Intercellular communication: diverse structures for exchange of genetic information. Nat. Rev. Mol. Cell Biol. 13, 328–335 (2012).
Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 (2007).
Skog, J. et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 10, 1470–1476 (2008).
Mittelbrunn, M. et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2, 282 (2011).
Montecalvo, A. et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood 119, 756–766 (2012).
Meckes, D.G. Jr. et al. Human tumor virus utilizes exosomes for intercellular communication. Proc. Natl. Acad. Sci. USA 107, 20370–20375 (2010).
Pegtel, D.M. et al. Functional delivery of viral miRNAs via exosomes. Proc. Natl. Acad. Sci. USA 107, 6328–6333 (2010).
Mozes, L.W. Lack of interferon-induced transfer of viral resistance against murine leukemia virus or poliovirus in cocultivated cells. Virology 116, 359–362 (1982).
Seeger, C. & Mason, W.S. Hepatitis B virus biology. Microbiol. Mol. Biol. Rev. 64, 51–68 (2000).
Shepard, C.W., Simard, E.P., Finelli, L., Fiore, A.E. & Bell, B.P. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol. Rev. 28, 112–125 (2006).
Kwon, H. & Lok, A.S. Hepatitis B therapy. Nat. Rev. Gastroenterol. Hepatol. 8, 275–284 (2011).
Li, J. et al. Inhibition of hepatitis B virus replication by MyD88 involves accelerated degradation of pregenomic RNA and nuclear retention of pre-S/S RNAs. J. Virol. 84, 6387–6399 (2010).
Hiraga, N. et al. Absence of viral interference and different susceptibility to interferon between hepatitis B virus and hepatitis C virus in human hepatocyte chimeric mice. J. Hepatol. 51, 1046–1054 (2009).
Lutgehetmann, M. et al. Hepatitis B virus limits response of human hepatocytes to interferon-α in chimeric mice. Gastroenterology 140, 2074–2083 (2011).
Chen, J. et al. Hepatitis B virus polymerase impairs interferon-(-induced STA T activation through inhibition of importin-(5 and protein kinase C-δ. Hepatology 57, 470–482 (2013).
Gao, B., Jeong, W.I. & Tian, Z. Liver: An organ with predominant innate immunity. Hepatology 47, 729–736 (2008).
Ishibashi, H., Nakamura, M., Komori, A., Migita, K. & Shimoda, S. Liver architecture, cell function, and disease. Semin. Immunopathol. 31, 399–409 (2009).
Wu, M. et al. Hepatitis B virus polymerase inhibits the interferon-inducible MyD88 promoter by blocking nuclear translocation of Stat1. J. Gen. Virol. 88, 3260–3269 (2007).
Yang, P.L., Althage, A., Chung, J. & Chisari, F.V. Hydrodynamic injection of viral DNA: a mouse model of acute hepatitis B virus infection. Proc. Natl. Acad. Sci. USA 99, 13825–13830 (2002).
Ke, B. et al. Adoptive transfer of ex vivo HO-1 modified bone marrow-derived macrophages prevents liver ischemia and reperfusion injury. Mol. Ther. 18, 1019–1025 (2010).
Trajkovic, K. et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319, 1244–1247 (2008).
Kosaka, N. et al. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J. Biol. Chem. 285, 17442–17452 (2010).
Dreux, M. et al. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe 12, 558–570 (2012).
Bianco, F. et al. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 28, 1043–1054 (2009).
Zhang, J., Reedy, M.C., Hannun, Y.A. & Obeid, L.M. Inhibition of caspases inhibits the release of apoptotic bodies: Bcl-2 inhibits the initiation of formation of apoptotic bodies in chemotherapeutic agent-induced apoptosis. J. Cell Biol. 145, 99–108 (1999).
Ostrowski, M. et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol 12, 19–30 (2010).
Thery, C., Amigorena, S., Raposo, G. & Clayton, A. in Current Protocols Cell Biology Ch 3, Unit 3, 22 (John Wiley & Sons, 2006).
Meckes, D.G. Jr. & Raab-Traub, N. Microvesicles and viral infection. J. Virol. 85, 12844–12854 (2011).
Turelli, P., Mangeat, B., Jost, S., Vianin, S. & Trono, D. Inhibition of hepatitis B virus replication by APOBEC3G. Science 303, 1829 (2004).
Park, I.H., Baek, K.W., Cho, E.Y. & Ahn, B.Y. PKR-dependent mechanisms of interferon-α for inhibiting hepatitis B virus replication. Mol. Cells 32, 167–172 (2011).
Keller, S. et al. Systemic presence and tumor-growth promoting effect of ovarian carcinoma released exosomes. Cancer Lett. 278, 73–81 (2009).
Weiss, S.R. & Navas-Martin, S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. 69, 635–664 (2005).
DuPage, M., Dooley, A.L. & Jacks, T. Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat. Protoc. 4, 1064–1072 (2009).
Takahashi, K. et al. Plasmacytoid dendritic cells sense hepatitis C virus-infected cells, produce interferon, and inhibit infection. Proc. Natl. Acad. Sci. USA 107, 7431–7436 (2010).
Record, M., Subra, C., Silvente-Poirot, S. & Poirot, M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem. Pharmacol. 81, 1171–1182 (2011).
Gibbings, D.J., Ciaudo, C., Erhardt, M. & Voinnet, O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat. Cell Biol. 11, 1143–1149 (2009).
Khatua, A.K., Taylor, H.E., Hildreth, J.E. & Popik, W. Exosomes packaging APOBEC3G confer human immunodeficiency virus resistance to recipient cells. J. Virol. 83, 512–521 (2009).
Chang, J., Block, T.M. & Guo, J.T. The innate immune response to hepatitis B virus infection: implications for pathogenesis and therapy. Antiviral Res. 96, 405–413 (2012).
Mao, R. et al. Indoleamine 2,3-dioxygenase mediates the antiviral effect of gamma interferon against hepatitis B virus in human hepatocyte-derived cells. J. Virol. 85, 1048–1057 (2011).
Xi, W. et al. Roles of TIPE2 in hepatitis B virus-induced hepatic inflammation in humans and mice. Mol. Immunol. 48, 1203–1208 (2011).
Diamond, M.S. & Farzan, M. The broad-spectrum antiviral functions of IFIT and IFITM proteins. Nat. Rev. Immunol. 13, 46–57 (2013).
Pinkert, C.A., Ornitz, D.M., Brinster, R.L. & Palmiter, R.D. An albumin enhancer located 10 kb upstream functions along with its promoter to direct efficient, liver-specific expression in transgenic mice. Genes Dev. 1, 268–276 (1987).
Zhang, D.E., Hetherington, C.J., Chen, H.M. & Tenen, D.G. The macrophage transcription factor PU.1 directs tissue-specific expression of the macrophage colony-stimulating factor receptor. Mol. Cell. Biol. 14, 373–381 (1994).
Fadel, B.M., Boutet, S.C. & Quertermous, T. Octamer-dependent in vivo expression of the endothelial cell-specific TIE2 gene. J. Biol. Chem. 274, 20376–20383 (1999).
Auwerx, J. The Human leukemia-cell line, Thp-1—a multifaceted model for the study of monocyte-macrophage differentiation. Experientia 47, 22–31 (1991).
Köck, J. & Blum, H.E. Hypermutation of hepatitis B virus genomes by APOBEC3G, APOBEC3C and APOBEC3H. J. Gen. Virol. 89, 1184–1191 (2008).
Liu, W. et al. Sample preparation method for isolation of single-cell types from mouse liver for proteomic studies. Proteomics 11, 3556–3564 (2011).
Acknowledgements
We thank C. Dong, N. Weng, J. Guo, J. Hu, S. Zhou and Q. Cai for suggestions and critical reading of the manuscript. Supported by the National Key Basic Research Program of China (2012CB519000 to Z.Y.), the National Megaprojects of China for Infectious Diseases (2012ZX10002007-001 to Z.Y.), the German Research Foundation (SFB/Transregio TRR60 to Z.Y.), the National Natural Science Foundation of China (31200129 to J. Li) and the China Postdoctoral Science Foundation (201104232 to J. Li).
Author information
Authors and Affiliations
Contributions
J. Li conceived of the study, designed the experiments, did most of the experiments and wrote the manuscript; K.L. isolated, characterized and labeled exosomes; Y.L. did quantitative RT-PCR; Y.X. and T.P. contributed to the HCV-replication assays; F.Z. generated the lentiviral expression vectors; H.Y. and X.Z. assisted in the hydrodynamic injections; J. Liu provided reagents; J.C. and M.W. helped with depletion and reconstitution of macrophages; and Z.Y. supervised all aspects of the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures and Text
Supplementary Figures 1–9, Supplementary Table 1 legend, and Supplementary Tables 2 and 3 (PDF 843 kb)
Supplementary Table 1
Differentially expressed mRNAs and miRNA in the exosomes from IFN-α-stimulated and unstimulated LSECs by microarray (XLS 462 kb)
Rights and permissions
About this article
Cite this article
Li, J., Liu, K., Liu, Y. et al. Exosomes mediate the cell-to-cell transmission of IFN-α-induced antiviral activity. Nat Immunol 14, 793–803 (2013). https://doi.org/10.1038/ni.2647
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2647
This article is cited by
-
Exosomal lncRNA XIST promotes perineural invasion of pancreatic cancer cells via miR-211-5p/GDNF
Oncogene (2024)
-
Engineered small extracellular vesicles as a novel platform to suppress human oncovirus-associated cancers
Infectious Agents and Cancer (2023)
-
Exosomal transmission of viruses, a two-edged biological sword
Cell Communication and Signaling (2023)
-
Endothelial cell CD36 regulates membrane ceramide formation, exosome fatty acid transfer and circulating fatty acid levels
Nature Communications (2023)
-
Proteomic profiling of urinary small extracellular vesicles in children with pneumonia: a pilot study
Pediatric Research (2023)