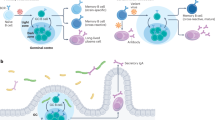
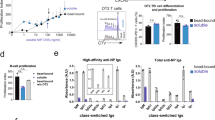
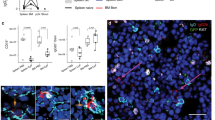
Abstract
Memory B cells are at the center of longstanding controversies regarding the presence of antigen for their survival and their re-engagement in germinal centers after secondary challenge. Using a new mouse model of memory B cell labeling dependent on the cytidine deaminase AID, we show that after immunization with a particulate antigen, B cell memory appeared in several subsets, comprising clusters of immunoglobulin M–positive (IgM+) and IgG1+ B cells in germinal center–like structures that persisted up to 8 months after immunization, as well as IgM+ and IgG1+ B cells with a memory phenotype outside of B cell follicles. After challenge, the IgG subset differentiated into plasmocytes, whereas the IgM subset reinitiated a germinal center reaction. This model, in which B cell memory appears in several layers with different functions, reconciles previous conflicting propositions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Plotkin, S. Vaccines: correlates of vaccine-induced immunity. Clin. Infect. Dis. 47, 401–409 (2008).
Berek, C., Berger, A. & Apel, M. Maturation of the immune response in germinal centers. Cell 67, 1121–1129 (1991).
Jacob, J., Kelsoe, G., Rajewsky, K. & Weiss, U. Intraclonal generation of antibody mutants in germinal centres. Nature 354, 389–392 (1991).
Muramatsu, M. et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102, 553–563 (2000).
Revy, P. et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the hyper-IgM syndrome (HIGM2). Cell 102, 565–575 (2000).
MacLennan, I.C., Liu, Y.J., Oldfield, S., Zhang, J. & Lane, P.J. The evolution of B-cell clones. Curr. Top. Microbiol. Immunol. 159, 37–63 (1990).
McHeyzer-Williams, L.J. & McHeyzer-Williams, M.G. Antigen-specific memory B cell development. Annu. Rev. Immunol. 23, 487–513 (2005).
Dorner, T. & Radbruch, A. Antibodies and B cell memory in viral immunity. Immunity 27, 384–392 (2007).
Mandel, T.E., Phipps, R.P., Abbot, A.P. & Tew, J.G. Long-term antigen retention by dendritic cells in the popliteal lymph node of immunized mice. Immunology 43, 353–362 (1981).
Gray, D. & Skarvall, H. B-cell memory is short-lived in the absence of antigen. Nature 336, 70–73 (1988).
Bachmann, M.F., Odermatt, B., Hengartner, H. & Zinkernagel, R.M. Induction of long-lived germinal centers associated with persisting antigen after viral infection. J. Exp. Med. 183, 2259–2269 (1996).
Schittek, B. & Rajewsky, K. Maintenance of B-cell memory by long-lived cells generated from proliferating precursors. Nature 346, 749–751 (1990).
Maruyama, M., Lam, K.P. & Rajewsky, K. Memory B-cell persistence is independent of persisting immunizing antigen. Nature 407, 636–642 (2000).
Siekevitz, M., Kocks, C., Rajewsky, K. & Dildrop, R. Analysis of somatic mutation and class switching in naive and memory B cells generating adoptive primary and secondary responses. Cell 48, 757–770 (1987).
Berek, C. & Milstein, C. Mutation drift and repertoire shift in the maturation of the immune response. Immunol. Rev. 96, 23–41 (1987).
Ridderstad, A. & Tarlinton, D.M. Kinetics of establishing the memory B cell population as revealed by CD38 expression. J. Immunol. 160, 4688–4695 (1998).
Takahashi, Y., Ohta, H. & Takemori, T. Fas is required for clonal selection in germinal centers and the subsequent establishment of the memory B cell repertoire. Immunity 14, 181–192 (2001).
Anderson, S.M., Tomayko, M.M., Ahuja, A., Haberman, A.M. & Shlomchik, M.J. New markers for murine memory B cells that define mutated and unmutated subsets. J. Exp. Med. 204, 2103–2114 (2007).
Smith, K.G., Light, A., Nossal, G.J. & Tarlinton, D.M. The extent of affinity maturation differs between the memory and antibody-forming cell compartments in the primary immune response. EMBO J. 16, 2996–3006 (1997).
Shinall, S.M., Gonzalez-Fernandez, M., Noelle, R.J. & Waldschmidt, T.J. Identification of murine germinal center B cell subsets defined by the expression of surface isotypes and differentiation antigens. J. Immunol. 164, 5729–5738 (2000).
Wolniak, K.L., Noelle, R.J. & Waldschmidt, T.J. Characterization of (4-hydroxy-3-nitrophenyl)acetyl (NP)-specific germinal center B cells and antigen-binding B220- cells after primary NP challenge in mice. J. Immunol. 177, 2072–2079 (2006).
Kaisho, T., Schwenk, F. & Rajewsky, K. The roles of γ1 heavy chain membrane expression and cytoplasmic tail in IgG1 responses. Science 276, 412–415 (1997).
Martin, S.W. & Goodnow, C.C. Burst-enhancing role of the IgG membrane tail as a molecular determinant of memory. Nat. Immunol. 3, 182–188 (2002).
Wakabayashi, C., Adachi, T., Wienands, J. & Tsubata, T. A distinct signaling pathway used by the IgG-containing B cell antigen receptor. Science 298, 2392–2395 (2002).
Feil, R., Wagner, J., Metzger, D. & Chambon, P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem. Biophys. Res. Commun. 237, 752–757 (1997).
Srinivas, S. et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 (2001).
Blink, E.J. et al. Early appearance of germinal center-derived memory B cells and plasma cells in blood after primary immunization. J. Exp. Med. 201, 545–554 (2005).
Breitfeld, D. et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 192, 1545–1552 (2000).
Schaerli, P. et al. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 192, 1553–1562 (2000).
Fazilleau, N. et al. Lymphoid reservoirs of antigen-specific memory T helper cells. Nat. Immunol. 8, 753–761 (2007).
Oliver, A.M., Martin, F. & Kearney, J.F. Mouse CD38 is down-regulated on germinal center B cells and mature plasma cells. J. Immunol. 158, 1108–1115 (1997).
Reichert, R.A., Gallatin, W.M., Weissman, I.L. & Butcher, E.C. Germinal center B cells lack homing receptors necessary for normal lymphocyte recirculation. J. Exp. Med. 157, 813–827 (1983).
Gatto, D. et al. Regulation of memory antibody levels: the role of persisting antigen versus plasma cell life span. J. Immunol. 178, 67–76 (2007).
McBride, K.M. et al. Regulation of class switch recombination and somatic mutation by AID phosphorylation. J. Exp. Med. 205, 2585–2594 (2008).
Takizawa, M. et al. AID expression levels determine the extent of cMyc oncogenic translocations and the incidence of B cell tumor development. J. Exp. Med. 205, 1949–1957 (2008).
White, H. & Gray, D. Analysis of immunoglobulin (Ig) isotype diversity and IgM/D memory in the response to phenyl-oxazolone. J. Exp. Med. 191, 2209–2220 (2000).
Williams, G.T., Jolly, C.J., Kohler, J. & Neuberger, M.S. The contribution of somatic hypermutation to the diversity of serum immunoglobulin: dramatic increase with age. Immunity 13, 409–417 (2000).
Richard, K., Pierce, S.K. & Song, W. The agonists of TLR4 and 9 are sufficient to activate memory B cells to differentiate into plasma cells in vitro but not in vivo. J. Immunol. 181, 1746–1752 (2008).
Weill, J.C., Weller, S. & Reynaud, C.A. Human marginal zone B cells. Annu. Rev. Immunol. 27, 267–285 (2009).
Berek, C. The development of B cells and the B-cell repertoire in the microenvironment of the germinal center. Immunol. Rev. 126, 5–19 (1992).
McHeyzer-Williams, M.G., Nossal, G.J. & Lalor, P.A. Molecular characterization of single memory B cells. Nature 350, 502–505 (1991).
Mamani-Matsuda, M. et al. The human spleen is a major reservoir for long-lived vaccinia virus-specific memory B cells. Blood 111, 4653–4659 (2008).
Delbos, F. et al. Contribution of DNA polymerase eta to immunoglobulin gene hypermutation in the mouse. J. Exp. Med. 201, 1191–1196 (2005).
Acknowledgements
We thank D. Metzger (Institute of Genetics and Molecular and Cellular Biology) for vector containing Cre-ERT2; P. Charnay (Institut National de la Santé et de la Recherche Médicale U368) for Rosa26-loxP-EYFP mice on a mixed C57BL/6 × DBA/2 background; A. De Smet for assistance with embryonic stem cell culture; D. Lecoeuche for technical help; the Service d'Expérimentation Animale et de Transgénèse (Villejuif) for the production of the AID-CreERT2 mouse line; the Necker Laboratoire d'Expérimentation Animale et de Transgénèse for maintaining mouse lines (V. Caulet, Y. Lepage, Y. Moreau and A. David); M. Garfa-Traoré and N. Goudin for training in confocal microscopy; and C. Berek and A. Cumano for critical reading of the manuscript. Supported by the Agence National de la Recherche (Microbiologie et Immunologie 'Immune Memory'), the Fondation Princesse Grace and the Ligue Contre le Cancer (équipe labellisée).
Author information
Authors and Affiliations
Contributions
I.D. designed and did most of the experiments and contributed to the manuscript; B.B. contributed to mouse cell analysis; V.V. and F.D. contributed to sequence analysis; J.M. sorted cells; S.S. contributed to the conception of the model; and C.-A.R and J.-C.W. share senior authorship and scientific responsibility.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Tables 1–2 (PDF 661 kb)
Rights and permissions
About this article
Cite this article
Dogan, I., Bertocci, B., Vilmont, V. et al. Multiple layers of B cell memory with different effector functions. Nat Immunol 10, 1292–1299 (2009). https://doi.org/10.1038/ni.1814
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1814
This article is cited by
-
Memory B cells
Nature Reviews Immunology (2024)
-
Tango of B cells with T cells in the making of secretory antibodies to gut bacteria
Nature Reviews Gastroenterology & Hepatology (2023)
-
Randomized, double-blind, placebo-controlled trial of rapamycin in amyotrophic lateral sclerosis
Nature Communications (2023)
-
Preclinical models for prediction of immunotherapy outcomes and immune evasion mechanisms in genetically heterogeneous multiple myeloma
Nature Medicine (2023)
-
Antibodies against endogenous retroviruses promote lung cancer immunotherapy
Nature (2023)