Abstract
Small cell carcinoma of the ovary, hypercalcemic type (SCCOHT) is the most common undifferentiated ovarian malignancy in women under 40 years of age1. We sequenced the exomes of six individuals from three families with SCCOHT. After discovering segregating deleterious germline mutations in SMARCA4 in all three families, we tested DNA from a fourth affected family, which also carried a segregating SMARCA4 germline mutation. All the familial tumors sequenced harbored either a somatic mutation or loss of the wild-type allele. Immunohistochemical analysis of these cases and additional familial and non-familial cases showed loss of SMARCA4 (BRG1) protein in 38 of 40 tumors overall. Sequencing of cases with available DNA identified at least one germline or somatic deleterious SMARCA4 mutation in 30 of 32 cases. Additionally, the SCCOHT cell line BIN-67 had biallelic deleterious mutations in SMARCA4. Our findings identify alterations in SMARCA4 as the major cause of SCCOHT, which could lead to improvements in genetic counseling and new treatment approaches.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Accession codes
References
Scully, R.E. in Atlas of Tumor Pathology (eds. Hartmann, W.H. & Cowan, W.R.) (Armed Forces Institute of Pathology, Washington, DC, 1979).
Eichhorn, J.H., Young, R.H. & Scully, R.E. Primary ovarian small cell carcinoma of pulmonary type. A clinicopathologic, immunohistologic, and flow cytometric analysis of 11 cases. Am. J. Surg. Pathol. 16, 926–938 (1992).
Clement, P.B. Selected miscellaneous ovarian lesions: small cell carcinomas, mesothelial lesions, mesenchymal and mixed neoplasms, and non-neoplastic lesions. Mod. Pathol. 18 (suppl. 2), S113–S129 (2005).
McCluggage, W.G.W. Ovarian neoplasms composed of small round cells: a review. Adv. Anat. Pathol. 11, 288–296 (2004).
McCluggage, W.G., Oliva, E., Connolly, L.E., McBride, H.A. & Young, R.H. An immunohistochemical analysis of ovarian small cell carcinoma of hypercalcemic type. Int. J. Gynecol. Pathol. 23, 330–336 (2004).
Young, R.H., Oliva, E. & Scully, R.E. Small cell carcinoma of the ovary, hypercalcemic type. A clinicopathological analysis of 150 cases. Am. J. Surg. Pathol. 18, 1102–1116 (1994).
Estel, R., Hackethal, A., Kalder, M. & Münstedt, K. Small cell carcinoma of the ovary of the hypercalcaemic type: an analysis of clinical and prognostic aspects of a rare disease on the basis of cases published in the literature. Arch. Gynecol. Obstet. 284, 1277–1282 (2011).
Ulbright, T.M., Roth, L.M., Stehman, F.B., Talerman, A. & Senekjian, E.K. Poorly differentiated (small cell) carcinoma of the ovary in young women: evidence supporting a germ cell origin. Hum. Pathol. 18, 175–184 (1987).
Peccatori, F., Bonazzi, C., Lucchini, V., Bratina, G. & Mangioni, C. Primary ovarian small cell carcinoma: four more cases. Gynecol. Oncol. 49, 95–99 (1993).
Lamovec, J., Bracko, M. & Cerar, O. Familial occurrence of small-cell carcinoma of the ovary. Arch. Pathol. Lab. Med. 119, 523–527 (1995).
Longy, M., Toulouse, C., Mage, P., Chauvergne, J. & Trojani, M. Familial cluster of ovarian small cell carcinoma: a new mendelian entity? J. Med. Genet. 33, 333–335 (1996).
Distelmaier, F. et al. Ovarian small cell carcinoma of the hypercalcemic type in children and adolescents. Cancer 107, 2298–2306 (2006).
Martinez-Borges, A.R. et al. Familial small cell carcinoma of the ovary. Pediatr. Blood Cancer 53, 1334–1336 (2009).
McDonald, J.M. et al. Small cell carcinoma of the ovary of hypercalcemic type: a case report. J. Pediatr. Surg. 47, 588–592 (2012).
Van Allen, E.M. et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov. 4, 94–109 (2014).
Holley, T. et al. Deep clonal profiling of formalin fixed paraffin embedded clinical samples. PLoS ONE 7, e50586 (2012).
Shain, A.H. & Pollack, J.R. The spectrum of SWI/SNF mutations, ubiquitous in human cancers. PLoS ONE 8, e55119 (2013).
Kadoch, C. et al. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat. Genet. 45, 592–601 (2013).
Wilson, B.G. & Roberts, C.W. SWI/SNF nucleosome remodellers and cancer. Nat. Rev. Cancer 11, 481–492 (2011).
Kosho, T. et al. Clinical correlations of mutations affecting six components of the SWI/SNF complex: detailed description of 21 patients and a review of the literature. Am. J. Med. Genet. A. 161A, 1221–1237 (2013).
Wong, A.K. et al. BRG1, a component of the SWI-SNF complex, is mutated in multiple human tumor cell lines. Cancer Res. 60, 6171–6177 (2000).
Gunduz, E. et al. Genetic and epigenetic alterations of BRG1 promote oral cancer development. Int. J. Oncol. 26, 201–210 (2005).
Sun, A. et al. Aberrant expression of SWI/SNF catalytic subunits BRG1/BRM is associated with tumor development and increased invasiveness in prostate cancers. Prostate 67, 203–213 (2007).
Richter, J. et al. Recurrent mutation of the ID3 gene in Burkitt lymphoma identified by integrated genome, exome and transcriptome sequencing. Nat. Genet. 44, 1316–1320 (2012).
Smith, M. et al. Frequency of SMARCB1 mutations in familial and sporadic schwannomatosis. Neurogenetics 13, 141–145 (2012).
Smith, M.J. et al. Loss-of-function mutations in SMARCE1 cause an inherited disorder of multiple spinal meningiomas. Nat. Genet. 45, 295–298 (2013).
Hasselblatt, M. et al. Nonsense mutation and inactivation of SMARCA4 (BRG1) in an atypical teratoid/rhabdoid tumor showing retained SMARCB1 (INI1) expression. Am. J. Surg. Pathol. 35, 933–935 (2011).
Schneppenheim, R. et al. Germline nonsense mutation and somatic inactivation of SMARCA4/BRG1 in a family with rhabdoid tumor predisposition syndrome. Am. J. Hum. Genet. 86, 279–284 (2010).
Witkowski, L. et al. Familial rhabdoid tumour 'avant la lettre'—from pathology review to exome sequencing and back again. J. Pathol. 231, 35–43 (2013).
Brennan, B., Stiller, C. & Bourdeaut, F. Extracranial rhabdoid tumours: what we have learned so far and future directions. Lancet Oncol. 14, e329–e336 (2013).
Pomeroy, S.L. et al. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature 415, 436–442 (2002).
Matsubara, D. et al. Lung cancer with loss of BRG1/BRM, shows epithelial mesenchymal transition phenotype and distinct histologic and genetic features. Cancer Sci. 104, 266–273 (2013).
Scully, R.E. Classification of human ovarian tumors. Environ. Health Perspect. 73, 15–25 (1987).
Kupryjańczyk, J. et al. Ovarian small cell carcinoma of hypercalcemic type—evidence of germline origin and SMARCA4 gene inactivation. A pilot study. Pol. J. Pathol. 64, 238–246 (2013).
Lee, R.S. et al. A remarkably simple genome underlies highly malignant pediatric rhabdoid cancers. J. Clin. Invest. 122, 2983–2988 (2012).
Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 474, 609–615 (2011).
Oike, T. et al. A synthetic lethality–based strategy to treat cancers harboring a genetic deficiency in the chromatin remodeling factor BRG1. Cancer Res. 73, 5508–5518 (2013).
Wilson, B.G. et al. Residual complexes containing SMARCA2 (BRM) underlie the oncogenic drive of SMARCA4 (BRG1) mutation. Mol. Cell. Biol. 34, 1136–1144 (2014).
FIGO Committee on Gynecologic Oncology. Current FIGO staging for cancer of the vagina, fallopian tube, ovary, and gestational trophoblastic neoplasia. Int. J. Gynaecol. Obstet. 105, 3–4 (2009).
Upchurch, K.S., Parker, L.M., Scully, R.E. & Krane, S.M. Differential cyclic AMP responses to calcitonin among human ovarian carcinoma cell lines: a calcitonin-responsive line derived from a rare tumor type. J. Bone Miner. Res. 1, 299–304 (1986).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Wang, K., Li, M. & Hakonarson, H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38, e164 (2010).
Witkowski, L. et al. DICER1 hotspot mutations in non-epithelial gonadal tumours. Br. J. Cancer 109, 2744–2750 (2013).
Schwartzentruber, J. et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482, 226–231 (2012).
Robinson, J.T. et al. Integrative Genomics Viewer. Nat. Biotechnol. 29, 24–26 (2011).
Cerami, E. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 (2012).
Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 474, 609–615 (2011).
Tohda, H., Oikawa, A., Kudo, T. & Tachibana, T. A greatly simplified method of establishing B-lymphoblastoid cell lines. Cancer Res. 38, 3560–3562 (1978).
Gamwell, L.F. et al. Small cell ovarian carcinoma: genomic stability and responsiveness to therapeutics. Orphanet J. Rare Dis. 8, 33 (2013).
Ventura, R.A. et al. FISH analysis for the detection of lymphoma-associated chromosomal abnormalities in routine paraffin-embedded tissue. J. Mol. Diagn. 8, 141–151 (2006).
Acknowledgements
This work is dedicated to the memory of Georgia Enter and Latosha Durham. We thank K. Hill and the Small Cell Ovarian Cancer Foundation for help in recruitment to this study, B. Vanderhyden (University of Ottawa) for providing the BIN-67 cells, S. Croce and C.S. Choong for aiding in the collection of pathological samples, P.-O. Fiset for help with immunohistochemistry and N. Benlimame, R. Zühlke-Jenisch, C. Kemming, M. Leiße, S. Peetz-Dienhart, L. Raestrup, K. Schmidt and C. Theile for technical assistance. N. Jabado, I. Bah and members of the Foulkes laboratory provided helpful discussions. We also thank the McGill University and Génome Québec Innovation Centre for their cooperation, H. Pierce for her help with contacting family 1 and D. Samelak for her help with family 3. Equipment for immunohistochemistry analysis was made available through Sonderforschungsbereich Transregio (SFB TR) 128 (grant to T. Kuhlmann (Z1)). We received funding from the Jewish General Hospital Foundation and the Jodi Taiger Lazarus Fund (W.D.F.), the Fonds de Recherche du Québec–Santé (L.W.), KinderKrebsInitiative Buchholz/Holm-Seppensen (R.S.) and Interdisziplinären Zentrum für Klinische Forschung (IZKF) Münster (Ha3/016/11) (M.H.).
Author information
Authors and Affiliations
Contributions
L.W. collected samples, performed the experiments and wrote the manuscript. J.C.-Z., S.F. and J.N. carried out bioinformatics analyses and provided text and figures. S.A. and J.A. helped with pathological examination. N.H. oversaw the experiments. E.T., D.G., E.S., C.G., R.G.K., E.A.R., F.R.U., A.M., K.P., S.M.C., J.L., L.M.R., T.M.U., T.A.B., V.G., M.L., A.B., M.T. and C.J.R.S. provided samples and critical input. B.R., E.C. and M.W. aided in experiments. R.S.-S., F.P., A.S. and H.J.M. aided in sample collection. M.A. aided in sample preparation. I.N. and R.S. performed FISH analysis. W.G.M. and B.A.C. provided samples and were the reference pathologists. Y.R. oversaw the preparation of formalin-fixed, paraffin-embedded samples for whole-exome sequencing. M.H. performed all immunohistochemistry analyses. J.M. oversaw all bioinformatics analysis. W.D.F. designed the project, wrote the manuscript and oversaw all aspects of the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
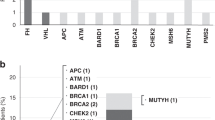
Supplementary Figure 1 Pedigrees of four families in which germline SMARCA4 mutations were found.
Pedigrees were not available for familial cases FA5 and FA6. In family 1, members II:2 and III:1 correspond to samples FA1a and FA1b, respectively, in Table 1. In family 2, members II:2 and III:2 correspond to samples FA2a and FA2b, respectively, in Table 1. In family 3, members II:2 and III:1 correspond to samples FA3a and FA3b, respectively, in Table 1. In family 4, members II:2 and III:1 correspond to samples FA4a and FA4b, respectively, in Table 1. SAB, spontaneous abortion; SCCOHT, small cell carcinoma of the ovary, hypercalcemic type; YST, yolk sac tumor; PSU, cancer, primary site unknown; +/+, wild-type for the familial SMARCA4 mutation; +/− , heterozygous for the familial SMARCA4 mutation. A diagonal line through a symbol indicates that the person is deceased. Whole-exome sequencing was carried out using DNA from individuals II:1 and III:1 (family 1); II:2 and III:2 (family 2); and II:1 and III:1 (family 3). The younger age of onset in recent generations in three of the four families is noted; it is likely to be mainly attributable to ascertainment bias. Individual II:4 from family 3 is at high risk for SCCOHT and has consented to a preventive bilateral oophorectomy.
Supplementary Figure 2 LOH analysis on the matched normal-tumor pair from FA1b.
LOH analysis on chromosome 19 for matched normal (FA1b(N)) and tumor (FA1b(T)) DNA from sample FA1b. BAF, B-allele frequency. See the Online Methods for details on LOH analysis. LOH was seen on chromosome 19p, where SMARCA4 is located, and not on 19q (or elsewhere in the genome; Supplementary Figure 4).
Supplementary Figure 3 SMARCA4 results from cases with and without SMARCA4 mutations.
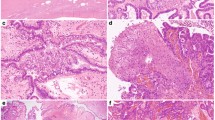
(a) Immunostaining results from familial cases. Chromatograms are shown in Figure 1 where available. (b) Immunostaining results and chromatograms for cases where mutations were found in non-familial cases and cases of unknown family history with SCCOHT, classic type. (c) Immunohistochemistry results and chromatograms of mutations from non-familial cases and cases of unknown family history with SCCOHT, large cell type. (d) Immunohistochemistry results and chromatograms of mutations for the group with unknown family history where the diagnoses were less certain and for non-SCCOHT cases. (e) Immunohistochemistry results for SCCOHT mimics. Note that SMARCA4 immunostaining is retained in all cases.
Supplementary Figure 4 Cycloheximide results for LCLs from family 3 member FA3b.
(a) Agarose gel of the cDNA PCR product amplified across the germline mutation c.2617−3C>G. Lane 1, cDNA from non-treated LCLs. Lane 2, cDNA from LCLs treated with cycloheximide to inhibit translation. Extra (larger) bands appear as parts of the intron are retained. The part of the intron that is retained is subsequently translated and codes for a premature stop codon, making the transcript subject to nonsense-mediated decay in non-treated cells. (b) Chromatogram showing the part of the intron retained as a result of the mutation. An asterisk denotes the mutation. (c) The amino acids encoded at the end of exon 18 are shown in black, and those encoded by the beginning of the retained intron are shown in red. After 12 amino acids, the intron codes for a stop codon, noted by an asterisk. This subjects the allele to nonsense-mediated decay.
Supplementary Figure 5 Genome-wide LOH analysis on SCCOHT samples.
Genome-wide LOH analysis was carried out for samples sequenced by whole-exome sequencing (Online Methods). Recurrent LOH is seen only on chromosome 19p, where SMARCA4 is located (shown in pink). The top three rows show tumor samples with no LOH on chromosome 19p. Rows 4 and 5 show the matched normal and tumor sample seen in Supplementary Figure 1, where row 4 is normal DNA and row 5 is matched tumor DNA with LOH on chromosome 19p. Rows 6−12 show samples with LOH on chromosome 19p, with the region of homozygosity represented in pink.
Supplementary Figure 6 Analysis from two cases that showed abnormal FISH results.
(a) Hematoxylin and eosin staining of UN11 and UN10. (b) SMARCA4 staining. (c) Interphase FISH analysis showing homozygous deletion of the SMARCA4 locus in cases UN11 and UN10 evaluated using Zeiss fluorescence microscopes equipped with appropriate filter sets and documented using ISIS software (MetaSystems, Altlusheim). The left panel for each case shows hybridization with the double-color (red and green) break-apart probe for SMARCA4, and the right panel shows hybridization of the same area with the control probe for the centromere of chromosome 6 (CEP6 labeled in blue, false color display as green to contrast nuclear counterstain in DAPI). Both cases show a significant number of cells lacking SMARCA4 signals but clearly showing CEP6 signals indicating homozygous loss. (d) Chromatograms of the mutations found in both cases.
Supplementary Figure 7 BIN-67 analysis.
(a) Chromatogram of the splice-site mutation in genomic DNA from allele 1. An asterisk denotes the mutation. (b) Chromatogram of the splice-site mutation in genomic DNA from allele 2. An asterisk denotes the mutation. (c) Effect of the mutation on allele 1. Top, chromatograms of cDNA sequence, retaining the beginning of intron 15 and the end of intron 16. Only one allele, allele 1, is being amplified, as the mutation in allele 2 is not present at the end of intron 16 (asterisk). Bottom, diagram of primer location and the expected size of primers. The entire product including both introns was too large to amplify and is not seen on the gel in e. (d) Effect of the mutation on allele 2. Top, chromatogram of cDNA sequence, splicing out exons 15 and 16. Bottom, diagram of primer location and the expected product size. With two exons spliced out, the sequence is in frame with no amino acid changes but does splice out part of the SNF2_N domain, including the DEXDc domain, which is part of the ATPase domain (Figure 1). (e) Gel of BIN-67 results. Lane 1, GAPDH control. Lanes 2 and 3, PCR product from intronic primers 1 and 2 showing that, for allele 2, although the entire product was too large to amplify, the beginning of exon 16 and end of intron 17 are present. Lane 4, BIN-67 cells treated with cycloheximide and non-treated, showing that no larger fragment degraded by nonsense-mediated decay was recovered with cycloheximide. Lane 6, PCR product from control cDNA, showing the correct product size. S, sense; AS, antisense; CHX, cycloheximide; +Ctrl, positive control; Int, intronic primer.
Supplementary Figure 8 Immunoblot analysis of SMARCA4 and SMARCA2 in the BIN-67 cell line.
(a) SMARCA4 and SMARCA2 protein expression in the BIN-67 cell line. HEK 293T and HeLa cell lines were used as controls. No normal SMARCA4 protein was detected in BIN-67 cells as a result of the biallelic mutations (c.2438+1G>A and c.2439−2A>T). SMARCA2 protein expression was substantially decreased in the BIN-67 cell line compared to the HEK 293T and HeLa cell lines. β-actin was used as a loading control. (b) SMARCA2 cDNA expression by PCR seen on an agarose gel. Lane 1, BIN-67; lane 2, positive control; lane 3, negative control. See Supplementary Table 6 for the primers used.
Supplementary Figure 9 Chronology of the study.
SMARCA4 mutations were originally discovered through whole-exome sequencing of samples from three families. As DNA was unavailable for the affected mothers from families 1 and 3, DNA from the unaffected fathers was used instead. We looked for deleterious variants that were present in the affected daughter and were not present in the unaffected father. The rest of the study was carried out as shown.
Supplementary information
Supplementary Text and Figures
Supplementary Note, Supplementary Figures 1–9 and Supplementary Tables 1–9 (PDF 5036 kb)
Source data
Rights and permissions
About this article
Cite this article
Witkowski, L., Carrot-Zhang, J., Albrecht, S. et al. Germline and somatic SMARCA4 mutations characterize small cell carcinoma of the ovary, hypercalcemic type. Nat Genet 46, 438–443 (2014). https://doi.org/10.1038/ng.2931
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2931
This article is cited by
-
The role of chromatin remodeler SMARCA4/BRG1 in brain cancers: a potential therapeutic target
Oncogene (2023)
-
The SMARCA4R1157W mutation facilitates chromatin remodeling and confers PRMT1/SMARCA4 inhibitors sensitivity in colorectal cancer
npj Precision Oncology (2023)
-
Establishment and characterization of a novel cell line (SCCOHT-CH-1) and PDX models derived from Chinese patients of small cell ovarian carcinoma of the hypercalcemic type
Human Cell (2023)
-
Concurrent high-grade glioma with cavernous malformations and pathogenic variants in PDCD10 and SMARCA4
Child's Nervous System (2023)