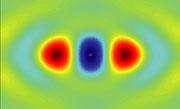
 The outermost electron in a nitrogen molecule is found around each of the atoms (shown in red and orange) and also contributes to the 'glue' between them (shown in blue).
The outermost electron in a nitrogen molecule is found around each of the atoms (shown in red and orange) and also contributes to the 'glue' between them (shown in blue).Chemistry is all about how electrons move around. Every reaction shifts fuzzy, quantum clouds of electrons from one place to another. So a technique unveiled today, which takes pictures of these clouds, could revolutionize our understanding of the molecules that surround us.
"This development marks a new epoch in our understanding of chemistry," says Jonathan Underwood, a physicist at the Open University, Milton Keynes, UK. "This technique is a major breakthrough, because, for the first time, it allows physicists to record a three-dimensional image of the orbitals of electrons in molecules."
“This development marks a new epoch in our understanding of chemistry.”
Jonathan Underwood
Open University, Milton Keynes, UK.
Molecules form when atoms team up, glued together by electrons that smear themselves into orbitals, which define the area where each electron is most likely to be found. When these molecules react, the electrons shift their allegiances between different atoms and change the shape of the molecular orbitals. This reshaping underpins all chemistry.
"The ability to observe a molecular orbital really caught a lot of people by surprise," says David Villeneuve, a physicist from the National Research Council of Canada's Steacie Institute for Molecular Sciences in Ottawa. Villeneuve was part of the Canadian and Japanese collaboration that invented the method, described in this week's Nature1. "An approximate take on quantum mechanics tells you that you can't directly observe an orbital, yet we have," he says.
Chemistry camera
The imaging technique uses extremely short laser pulses to briefly ionize an electron away from a molecule of nitrogen, which is simply two nitrogen atoms stuck together. As they spring back, the electrons emit light that can interfere with the laser pulse in different ways depending on the electron's position and where the laser pulse hit the molecule.
“An approximate take on quantum mechanics tells you that you can't directly observe an orbital, yet we have.”
David Villeneuve
Steacie Institute for Molecular Sciences, Ottawa, Canada.
Measuring this interference for thousands of ionizations allowed the scientists to reconstruct the shape of the outermost electron orbital in nitrogen. It produces a blurred image, says Villeneuve, like a swarm of flies snapped in a long-exposure picture.
The electron is pulled out of position by the laser pulse's oscillating electric field. This field cycles from high to low and back again every two femtoseconds (2x10-15 seconds), which is fast enough to catch electrons moving around during chemical reactions as well. "This technique could enable you to see how electrons rearrange as atoms approach each other," says Villeneuve.
Snapping bonds
Although the scientists have only looked at a simple, linear nitrogen molecule so far, Underwood has just started working with the team on a project to image the electrons around more complex molecules. Early results suggest that this will be possible, he says.
The technique could eventually help chemists to improve existing chemical reactions, design new catalysts or even understand how biological processes work. Protein folding, for example, relies on subtle interactions between atoms and electrons that many scientists are now trying to simulate with computers. The way a protein folds often determines how it acts in our bodies; in the case of new-variant Creutzfeldt-Jakob disease, incorrectly folded proteins can be lethal. The chemical camera could help to refine models to help us understand better why proteins fold in particular ways, suggests Villeneuve.
"In the near future, it should be possible to watch directly how the bonds of molecules change during chemical reactions," says Henrik Stapelfeldt, a physicist at the University of Aarhus, Langelandsgade, Denmark, "but this is already a spectacular achievement."
Open University, Milton Keynes, UK.
Steacie Institute for Molecular Sciences, Ottawa, Canada.
