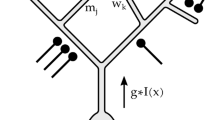
Abstract
Understanding a sensory system implies the ability to predict responses to a variety of inputs from a common model. In the retina, this includes predicting how the integration of signals across visual space shapes the outputs of retinal ganglion cells. Existing models of this process generalize poorly to predict responses to new stimuli. This failure arises in part from properties of the ganglion cell response that are not well captured by standard receptive-field mapping techniques: nonlinear spatial integration and fine-scale heterogeneities in spatial sampling. Here we characterize a ganglion cell's spatial receptive field using a mechanistic model based on measurements of the physiological properties and connectivity of only the primary excitatory circuitry of the retina. The resulting simplified circuit model successfully predicts ganglion-cell responses to a variety of spatial patterns and thus provides a direct correspondence between circuit connectivity and retinal output.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Chichilnisky, E.J. A simple white noise analysis of neuronal light responses. Network 12, 199–213 (2001).
Pillow, J.W. et al. Spatio-temporal correlations and visual signalling in a complete neuronal population. Nature 454, 995–999 (2008).
Pillow, J.W., Paninski, L., Uzzell, V.J., Simoncelli, E.P. & Chichilnisky, E.J. Prediction and decoding of retinal ganglion cell responses with a probabilistic spiking model. J. Neurosci. 25, 11003–11013 (2005).
Enroth-Cugell, C. & Robson, J.G. The contrast sensitivity of retinal ganglion cells of the cat. J. Physiol. (Lond.) 187, 517–552 (1966).
Caldwell, J.H. & Daw, N.W. New properties of rabbit retinal ganglion cells. J. Physiol. (Lond.) 276, 257–276 (1978).
Stone, C. & Pinto, L.H. Response properties of ganglion cells in the isolated mouse retina. Vis. Neurosci. 10, 31–39 (1993).
Petrusca, D. et al. Identification and characterization of a Y-like primate retinal ganglion cell type. J. Neurosci. 27, 11019–11027 (2007).
Demb, J.B., Haarsma, L., Freed, M.A. & Sterling, P. Functional circuitry of the retinal ganglion cell's nonlinear receptive field. J. Neurosci. 19, 9756–9767 (1999).
Hochstein, S. & Shapley, R.M. Linear and nonlinear spatial subunits in Y cat retinal ganglion cells. J. Physiol. (Lond.) 262, 265–284 (1976).
Victor, J.D. & Shapley, R.M. The nonlinear pathway of Y ganglion cells in the cat retina. J. Gen. Physiol. 74, 671–689 (1979).
Demb, J.B., Zaghloul, K., Haarsma, L. & Sterling, P. Bipolar cells contribute to nonlinear spatial summation in the brisk-transient (Y) ganglion cell in mammalian retina. J. Neurosci. 21, 7447–7454 (2001).
Gauthier, J.L. et al. Receptive fields in primate retina are coordinated to sample visual space more uniformly. PLoS Biol. 7, e1000063 (2009).
Thibos, L.N. & Levick, W.R. Bimodal receptive fields of cat retinal ganglion cells. Vision Res. 23, 1561–1572 (1983).
Passaglia, C.L., Troy, J.B., Ruttiger, L. & Lee, B.B. Orientation sensitivity of ganglion cells in primate retina. Vision Res. 42, 683–694 (2002).
Brown, S.P., He, S. & Masland, R.H. Receptive field microstructure and dendritic geometry of retinal ganglion cells. Neuron 27, 371–383 (2000).
Soo, F.S., Schwartz, G.W., Sadeghi, K. & Berry, M.J. II. Fine spatial information represented in a population of retinal ganglion cells. J. Neurosci. 31, 2145–2155 (2011).
Volgyi, B., Chheda, S. & Bloomfield, S.A. Tracer coupling patterns of the ganglion cell subtypes in the mouse retina. J. Comp. Neurol. 512, 664–687 (2009).
Pang, J.J., Gao, F. & Wu, S.M. Light-evoked excitatory and inhibitory synaptic inputs to ON and OFF alpha ganglion cells in the mouse retina. J. Neurosci. 23, 6063–6073 (2003).
Murphy, G.J. & Rieke, F. Network variability limits stimulus-evoked spike timing precision in retinal ganglion cells. Neuron 52, 511–524 (2006).
Gollisch, T. & Meister, M. Eye smarter than scientists believed: neural computations in circuits of the retina. Neuron 65, 150–164 (2010).
Schwartz, G.W. & Rieke, F. Nonlinear spatial encoding by retinal ganglion cells: when 1 + 1 ≠ 2. J. Gen. Physiol. 138, 283–290 (2011).
Wassle, H., Puller, C., Muller, F. & Haverkamp, S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J. Neurosci. 29, 106–117 (2009).
Yamagata, M. & Sanes, J.R. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature 451, 465–469 (2008).
Morgan, J.L., Soto, F., Wong, R.O. & Kerschensteiner, D. Development of cell type-specific connectivity patterns of converging excitatory axons in the retina. Neuron 71, 1014–1021 (2011).
Morgan, J.L., Schubert, T. & Wong, R.O. Developmental patterning of glutamatergic synapses onto retinal ganglion cells. Neural Dev. 3, 8 (2008).
Lin, B. & Masland, R.H. Synaptic contacts between an identified type of ON cone bipolar cell and ganglion cells in the mouse retina. Eur. J. Neurosci. 21, 1257–1270 (2005).
Kerschensteiner, D., Morgan, J.L., Parker, E.D., Lewis, R.M. & Wong, R.O. Neurotransmission selectively regulates synapse formation in parallel circuits in vivo. Nature 460, 1016–1020 (2009).
Berntson, A. & Taylor, W.R. Response characteristics and receptive field widths of on-bipolar cells in the mouse retina. J. Physiol. (Lond.) 524, 879–889 (2000).
Dacey, D. et al. Center surround receptive field structure of cone bipolar cells in primate retina. Vision Res. 40, 1801–1811 (2000).
Mills, S.L. & Massey, S.C. Differential properties of two gap junctional pathways made by AII amacrine cells. Nature 377, 734–737 (1995).
Freed, M.A., Smith, R.G. & Sterling, P. Computational model of the on-alpha ganglion cell receptive field based on bipolar cell circuitry. Proc. Natl. Acad. Sci. USA 89, 236–240 (1992).
Cohen, E. & Sterling, P. Microcircuitry related to the receptive field center of the on-beta ganglion cell. J. Neurophysiol. 65, 352–359 (1991).
Jakobs, T.C., Koizumi, A. & Masland, R.H. The spatial distribution of glutamatergic inputs to dendrites of retinal ganglion cells. J. Comp. Neurol. 510, 221–236 (2008).
Koizumi, A., Jakobs, T.C. & Masland, R.H. Regular mosaic of synaptic contacts among three retinal neurons. J. Comp. Neurol. 519, 341–357 (2011).
Zeck, G.M., Xiao, Q. & Masland, R.H. The spatial filtering properties of local edge detectors and brisk-sustained retinal ganglion cells. Eur. J. Neurosci. 22, 2016–2026 (2005).
Creutzfeldt, O.D., Sakmann, B., Scheich, H. & Korn, A. Sensitivity distribution and spatial summation within receptive-field center of retinal on-center ganglion cells and transfer function of the retina. J. Neurophysiol. 33, 654–671 (1970).
Kier, C.K., Buchsbaum, G. & Sterling, P. How retinal microcircuits scale for ganglion cells of different size. J. Neurosci. 15, 7673–7683 (1995).
Koch, C., Poggio, T. & Torre, V. Retinal ganglion cells: a functional interpretation of dendritic morphology. Phil. Trans. R. Soc. Lond. B 298, 227–263 (1982).
Bock, D.D. et al. Network anatomy and in vivo physiology of visual cortical neurons. Nature 471, 177–182 (2011).
Helmstaedter, M., Briggman, K.L. & Denk, W. High-accuracy neurite reconstruction for high-throughput neuroanatomy. Nat. Neurosci. 14, 1081–1088 (2011).
Hochstein, S. & Shapley, R.M. Quantitative analysis of retinal ganglion cell classifications. J. Physiol. (Lond.) 262, 237–264 (1976).
Badea, T.C. & Nathans, J. Quantitative analysis of neuronal morphologies in the mouse retina visualized by using a genetically directed reporter. J. Comp. Neurol. 480, 331–351 (2004).
Sun, W., Li, N. & He, S. Large-scale morphological survey of mouse retinal ganglion cells. J. Comp. Neurol. 451, 115–126 (2002).
Kong, J.H., Fish, D.R., Rockhill, R.L. & Masland, R.H. Diversity of ganglion cells in the mouse retina: unsupervised morphological classification and its limits. J. Comp. Neurol. 489, 293–310 (2005).
Coombs, J., van der List, D., Wang, G.Y. & Chalupa, L.M. Morphological properties of mouse retinal ganglion cells. Neuroscience 140, 123–136 (2006).
Masland, R.H. The fundamental plan of the retina. Nat. Neurosci. 4, 877–886 (2001).
Amthor, F.R., Takahashi, E.S. & Oyster, C.W. Morphologies of rabbit retinal ganglion cells with complex receptive fields. J. Comp. Neurol. 280, 97–121 (1989).
Kim, I.J., Zhang, Y., Yamagata, M., Meister, M. & Sanes, J.R. Molecular identification of a retinal cell type that responds to upward motion. Nature 452, 478–482 (2008).
Wassle, H., Peichl, L. & Boycott, B.B. Dendritic territories of cat retinal ganglion cells. Nature 292, 344–345 (1981).
Ala-Laurila, P., Greschner, M., Chichilnisky, E.J. & Rieke, F. Cone photoreceptor contributions to noise and correlations in the retinal output. Nat. Neurosci. 14, 1309–1316 (2011).
Abbott, L.F. & Dayan, P. The effect of correlated variability on the accuracy of a population code. Neural Comput. 11, 91–101 (1999).
Acknowledgements
We thank D. Perkel, G. Field, M. Berry and W. Bair for helpful comments on the manuscript. This research was made possible by support from the US National Institutes of Health (EY11850 to F.R., EY10699 and EY017101 to R.O.W. and the Vision Core Grant EY 01730), the Howard Hughes Medical Institute (F.R.) and the Helen Hay Whitney Foundation (G.W.S.).
Author information
Authors and Affiliations
Contributions
G.W.S. performed ganglion cell recordings, analysis and designed receptive-field models. H.O. performed imaging experiments and analyses (Figs. 4 and 6). F.A.D. performed bipolar cell recordings (Fig. 7b). J.L.M. and D.K. performed imaging experiments analyzed in Figure 6. G.W.S., R.O.W. and F.R. conceived of experiments and analyses. G.W.S., H.O., F.A.D., R.O.W. and F.R. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6, Supplementary Tables 1–3, Supplementary Discussion (PDF 3773 kb)
Supplementary Movie 1
Identifying appositions of PSD95 puncta with type 6 bipolar axon terminals. The dendritic segment shown in Figure 4c–e is zoomed in and rotated in three dimensions to demonstrate the identification of appositions with type 6 bipolar axon terminals. PSD95-CFP puncta, tdTomato filled alpha-like On RGC dendrites, On bipolar axons labeled by YFP and Syt2 immunoreactivity are shown in green, blue, red and white, respectively. The first set of flashing white dots represent all the identified PSD95 puncta and the second set show those apposed to On bipolar axon terminals. The red flashing dots represent PSD95 puncta classified as apposed to type 6 bipolar cells. (MOV 17179 kb)
Supplementary Movie 2
Identifying appositions of PSD95 puncta with type 7 bipolar axon terminals. The identification of the apposition of PSD95 puncta with type 7 bipolar cells is demonstrated in three dimensions in the same way as in Supplementary Movie 1 using the dendritic segment enlarged in Figure 4i,j. PSD95-CFP puncta, tdTomato filled alpha-like On RGC dendrites, type 7 bipolar axons labeled by GFP are shown in green, blue and red, respectively. The first set of flashing white dots represents all the identified PSD95 puncta and the second set represents those apposed to type 7 bipolar cells. (MOV 15646 kb)
Rights and permissions
About this article
Cite this article
Schwartz, G., Okawa, H., Dunn, F. et al. The spatial structure of a nonlinear receptive field. Nat Neurosci 15, 1572–1580 (2012). https://doi.org/10.1038/nn.3225
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3225
This article is cited by
-
Classical center-surround receptive fields facilitate novel object detection in retinal bipolar cells
Nature Communications (2022)
-
Suppression without inhibition: how retinal computation contributes to saccadic suppression
Communications Biology (2022)
-
Linear and nonlinear chromatic integration in the mouse retina
Nature Communications (2021)
-
An offset ON–OFF receptive field is created by gap junctions between distinct types of retinal ganglion cells
Nature Neuroscience (2021)
-
Type-specific dendritic integration in mouse retinal ganglion cells
Nature Communications (2020)