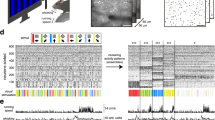
Abstract
Two intermingled hypothalamic neuron populations specified by expression of agouti-related peptide (AGRP) or pro-opiomelanocortin (POMC) positively and negatively influence feeding behavior, respectively, possibly by reciprocally regulating downstream melanocortin receptors. However, the sufficiency of these neurons to control behavior and the relationship of their activity to the magnitude and dynamics of feeding are unknown. To measure this, we used channelrhodopsin-2 for cell type–specific photostimulation. Activation of only 800 AGRP neurons in mice evoked voracious feeding within minutes. The behavioral response increased with photoexcitable neuron number, photostimulation frequency and stimulus duration. Conversely, POMC neuron stimulation reduced food intake and body weight, which required melanocortin receptor signaling. However, AGRP neuron–mediated feeding was not dependent on suppressing this melanocortin pathway, indicating that AGRP neurons directly engage feeding circuits. Furthermore, feeding was evoked selectively over drinking without training or prior photostimulus exposure, which suggests that AGRP neurons serve a dedicated role coordinating this complex behavior.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
25 February 2011
In the HTML version of this article initially published online, the date published was given as 5 January 2010. The correct date is 5 January 2011. The error has been corrected for all versions of this article
References
Morton, G.J., Cummings, D.E., Baskin, D.G., Barsh, G.S. & Schwartz, M.W. Central nervous system control of food intake and body weight. Nature 443, 289–295 (2006).
Ollmann, M.M. et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278, 135–138 (1997).
Clark, J.T., Kalra, P.S., Crowley, W.R. & Kalra, S.P. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology 115, 427–429 (1984).
Levine, A.S. & Morley, J.E. Neuropeptide Y: a potent inducer of consummatory behavior in rats. Peptides 5, 1025–1029 (1984).
Stanley, B.G. & Leibowitz, S.F. Neuropeptide Y: stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci. 35, 2635–2642 (1984).
Takahashi, K.A. & Cone, R.D. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology 146, 1043–1047 (2005).
Tsujii, S. & Bray, G.A. Acetylation alters the feeding response to MSH and beta-endorphin. Brain Res. Bull. 23, 165–169 (1989).
Yaswen, L., Diehl, N., Brennan, M.B. & Hochgeschwender, U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat. Med. 5, 1066–1070 (1999).
Cowley, M.A. et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411, 480–484 (2001).
Roseberry, A.G., Liu, H., Jackson, A.C., Cai, X. & Friedman, J.M. Neuropeptide Y-mediated inhibition of proopiomelanocortin neurons in the arcuate nucleus shows enhanced desensitization in ob/ob mice. Neuron 41, 711–722 (2004).
Cowley, M.A. et al. Electrophysiological actions of peripheral hormones on melanocortin neurons. Ann. NY Acad. Sci. 994, 175–186 (2003).
Luquet, S., Perez, F.A., Hnasko, T.S. & Palmiter, R.D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310, 683–685 (2005).
Wu, Q., Howell, M.P., Cowley, M.A. & Palmiter, R.D. Starvation after AgRP neuron ablation is independent of melanocortin signaling. Proc. Natl. Acad. Sci. USA 105, 2687–2692 (2008).
Wu, Q., Boyle, M.P. & Palmiter, R.D. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell 137, 1225–1234 (2009).
Boyden, E.S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Li, X. et al. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc. Natl. Acad. Sci. USA 102, 17816–17821 (2005).
Atasoy, D., Aponte, Y., Su, H.H. & Sternson, S.M.A. FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J. Neurosci. 28, 7025–7030 (2008).
Kaelin, C.B., Xu, A.W., Lu, X.Y. & Barsh, G.S. Transcriptional regulation of agouti-related protein (Agrp) in transgenic mice. Endocrinology 145, 5798–5806 (2004).
Balthasar, N. et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42, 983–991 (2004).
van den Top, M., Lee, K., Whyment, A.D., Blanks, A.M. & Spanswick, D. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat. Neurosci. 7, 493–494 (2004).
Tolkamp, B.J., Allcroft, D.J., Austin, E.J., Nielsen, B.L. & Kyriazakis, I.I. Satiety splits feeding behaviour into bouts. J. Theor. Biol. 194, 235–250 (1998).
Tolkamp, B.J. & Kyriazakis, I.I. To split behaviour into bouts, log-transform the intervals. Anim. Behav. 57, 807–817 (1999).
Miller, M.W. et al. Cloning of the mouse agouti gene predicts a secreted protein ubiquitously expressed in mice carrying the lethal yellow mutation. Genes Dev. 7, 454–467 (1993).
Kristensen, P. et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature 393, 72–76 (1998).
Elias, C.F. et al. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron 21, 1375–1385 (1998).
Rossi, M. et al. A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology 139, 4428–4431 (1998).
Fan, W., Boston, B.A., Kesterson, R.A., Hruby, V.J. & Cone, R.D. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385, 165–168 (1997).
Huber, D. et al. Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice. Nature 451, 61–64 (2008).
Houweling, A.R. & Brecht, M. Behavioural report of single neuron stimulation in somatosensory cortex. Nature 451, 65–68 (2008).
Tsai, H.C. et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324, 1080–1084 (2009).
Adamantidis, A.R., Zhang, F., Aravanis, A.M., Deisseroth, K. & de Lecea, L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature 450, 420–424 (2007).
Abbott, S.B. et al. Photostimulation of retrotrapezoid nucleus phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J. Neurosci. 29, 5806–5819 (2009).
Belgardt, B.F., Okamura, T. & Bruning, J.C. Hormone and glucose signaling in POMC and AgRP neurons. J. Physiol. (Lond.) 587, 5305–5314 (2009).
Broberger, C., Johansen, J., Johansson, C., Schalling, M. & Hokfelt, T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc. Natl. Acad. Sci. USA 95, 15043–15048 (1998).
Aravanis, A.M. et al. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J. Neural Eng. 4, S143–S156 (2007).
Peng, H., Ruan, Z., Long, F., Simpson, J.H. & Myers, E.W. V3D enables real-time 3D visualization and quantitative analysis of large-scale biological image data sets. Nat. Biotechnol. 28, 348–353 (2010).
Abercrombie, M. Estimation of nuclear population from microtome sections. Anat. Rec. 94, 239–247 (1946).
Holm, S.A. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6, 65–70 (1979).
Acknowledgements
We thank G. Shtengel for assistance with photostimulation equipment and software, R. Shusterman for assistance with data analysis, H. Peng for image analysis tools, J. Osborne and T. Tabachnik for equipment design and fabrication, A. Arnold for imaging support, B. Shields and A. Hu for histology support, and J. Cox for mouse breeding and genotyping support. K. Svoboda, G. Murphy, J. Dudman, A. Lee, and S.E.R. Egnor commented on the manuscript. This research was funded by the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
Y.A. performed the behavioral experiments and D.A. performed and analyzed electrophysiological experiments. Y.A. and S.M.S. designed the study, analyzed the data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 9261 kb)
Rights and permissions
About this article
Cite this article
Aponte, Y., Atasoy, D. & Sternson, S. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci 14, 351–355 (2011). https://doi.org/10.1038/nn.2739
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2739
This article is cited by
-
AgRP neurons encode circadian feeding time
Nature Neuroscience (2024)
-
Modulation of foraging-like behaviors by cholesterol-FGF19 axis
Cell & Bioscience (2023)
-
The melanocortin action is biased toward protection from weight loss in mice
Nature Communications (2023)
-
Neural basis for fasting activation of the hypothalamic–pituitary–adrenal axis
Nature (2023)
-
Increased intrinsic and synaptic excitability of hypothalamic POMC neurons underlies chronic stress-induced behavioral deficits
Molecular Psychiatry (2023)