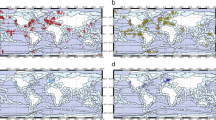
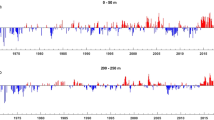
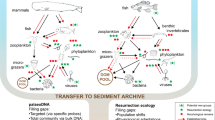
Abstract
Historical evidence provides essential context for models predicting the biological impacts of climate change. Such long-term data sets are relatively common for terrestrial taxa and environments, but sparse for aquatic systems. Aquatic biochronologies — generated from information recorded in the hard parts of fish, molluscs and corals that are archived in their millions worldwide — can provide valuable long-term ecological insights into marine and freshwater environments. These resources are, however, at present under-utilized in the measurement and prediction of ecological responses to climate change, despite their potential to provide unprecedented levels of spatial and temporal detail in aquatic environments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ozgul, A. et al. The dynamics of phenotypic change and the shrinking sheep of St. Kilda. Science 325, 464–467 (2009).
Penuelas, J., Filella, I. & Comas, P. Changed plant and animal life cycles from 1952 to 2000 in the Mediterranean region. Glob. Change Biol. 8, 531–544 (2002).
Jackson, J. B. C. et al. Historical overfishing and the recent collapse of coastal ecosystems. Science 293, 629–638 (2001).
Pyke, G. H. & Ehrlich, P. R. Biological collections and ecological/environmental research: A review, some observations and a look to the future. Biol. Rev. 85, 247–266 (2010).
Thackeray, S. J. et al. Trophic level asynchrony in rates of phenological change for marine, freshwater and terrestrial environments. Glob. Change Biol. 16, 3304–3313 (2010).
Southward, A. J. et al. Long-term oceanographic and ecological research in the western English Channel. Adv. Mar. Biol. 47, 1–105 (2005).
Field, D. B., Baumgartner, T. R., Charles, C. D., Ferreira-Bartrina, V. & Ohman, M. D. Planktonic foraminifera of the California Current reflect 20th-century warming. Science 311, 63–66 (2006).
Richardson, A. J. & Poloczanska, E. S. Ocean science under-resourced, under threat. Science 320, 1294–1295 (2008).
Brander, K., Bruno, J., Hobday, A. & Schoeman, D. The value of attribution. Nature Clim. Change 1, 70–71 (2011).
Brown, C. J. et al. Quantitative approaches in climate change ecology. Glob. Change Biol. 17, 3697–3713 (2011).
Campana, S. E. Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J. Fish Biol. 59, 197–242 (2001).
Kalish, J. M. Pre-bomb and post-bomb radiocarbon in fish otoliths. Earth Planet. Sci. Lett. 114, 549–554 (1993).
Knutson, D. W., Buddemeier, R. W. & Smith, S. V. Coral chronometers: Seasonal growth bands in reef corals. Science 177, 270–272 (1972).
Pannella, G. & MacClintock, C. Biological and environmental rhythms reflected in molluscan shell growth. Mem. J. Paleontol. 42, 64–80 (1968).
Campana, S. E. & Thorrold, S. R. Otoliths, increments, and elements: Keys to a comprehensive understanding of fish populations? Can. J. Fish. Aquat. Sci. 58, 30–38 (2001).
Morrongiello, J. R., Robertson, S. & Thresher, R. Australian Otolith Collections http://www.otolithcollections.net (2011).
Andrus, C. F. T. Shell midden sclerochronology. Quat. Sci. Rev. 30, 2892–2905 (2011).
Roark, E. B., Guilderson, T. P., Dunbar, R. B., Fallon, S. J. & Mucciarone, D. A. Extreme longevity in proteinaceous deep-sea corals. Proc. Natl Acad. Sci. USA 106, 5204–5208 (2009).
Tracey, D. M. & Horn, P. L. Background and review of ageing orange roughy (Hoplostethus atlanticus, Trachichthyidae) from New Zealand and elsewhere. NZ J. Mar. Freshwat. Res. 33, 67–86 (1999).
Kilada, R. W., Campana, S. E. & Roddick, D. Validated age, growth, and mortality estimates of the ocean quahog (Arctica islandica) in the western Atlantic. ICES J. Mar. Sci. 64, 31–38 (2007).
Druffel, E. R. M. Geochemistry of corals: Proxies of past ocean chemistry, ocean circulation, and climate. Proc. Natl Acad. Sci. USA 94, 8354–8361 (1997).
Lough, J. M. & Cooper, T. F. New insights from coral growth band studies in an era of rapid environmental change. Earth Sci. Rev. 108, 170–184 (2011).
Fritts, H. C. & Swetnam, T. W. Dendroecology: A tool for evaluating variations in past and present forest environments. Adv. Ecol. Res. 19, 111–188 (1989).
Gergis, J. L. & Fowler, A. M. A history of ENSO events since AD 1525: Implications for future climate change. Climatic Change 92, 343–387 (2009).
Cook, E. R. & Kairiukstis, L. A. Methods of Dendrochronology: Applications in the Environmental Sciences (Kluwer Academic, 1990).
Fritts, H. C. Tree Rings and Climate (Academic, 1976).
Lapointe-Garant, M. P. et al. Use of tree rings to study the effect of climate change on trembling aspen in Quebec. Glob. Change Biol. 16, 2039–2051 (2010).
Campana, S. E. How reliable are growth back-calculations based on otoliths? Can. J. Fish. Aquat. Sci. 47, 2219–2227 (1990).
Casselman, J. M. Growth and relative size of calcified structures of fish. Trans. Am. Fish. Soc. 119, 673–688 (1990).
Guyette, R. P. & Rabeni, C. F. Climate response among growth increments of fish and trees. Oecologia 104, 272–279 (1995).
Morrongiello, J. R., Crook, D. A., King, A. J., Ramsey, D. S. L. & Brown, P. Impacts of drought and predicted effects of climate change on fish growth in temperate Australian lakes. Glob. Change Biol. 17, 745–755 (2011).
Rowell, K. et al. Diverting the Colorado River leads to a dramatic life history shift in an endangered marine fish. Biol. Conserv. 141, 1138–1148 (2008).
Kendall, N. W., Rich, H. B., Jensen, L. R. & Quinn, T. P. Climate effects on inter-annual variation in growth of the freshwater mussel (Anodonta beringiana) in an Alaskan lake. Freshwat. Biol. 55, 2339–2346 (2010).
Black, B. A. et al. Winter and summer upwelling modes and their biological importance in the California Current ecosystem. Glob. Change Biol. 17, 2536–2545 (2011).
Carilli, J. E., Norris, R. D., Black, B., Walsh, S. M. & McField, M. Century-scale records of coral growth rates indicate that local stressors reduce coral thermal tolerance threshold. Glob. Change Biol. 16, 1247–1257 (2010).
Ostazeski, J. J. & Spangler, G. R. Use of biochronology to examine interactions of freshwater drum, walleye and yellow perch in the Red Lakes of Minnesota. Environ. Biol. Fish. 61, 381–393 (2001).
De'ath, G., Lough, J. M. & Fabricius, K. E. Declining coral calcification on the Great Barrier Reef. Science 323, 116–119 (2009).
Neuheimer, A. B., Thresher, R. E., Lyle, J. M. & Semmens, J. M. Tolerance limit for fish growth exceeded by warming waters. Nature Clim. Change 1, 110–113 (2011).
Cooper, T. F., O'Leary, R. A. & Lough, J. M. Growth of Western Australian corals in the Anthropocene. Science 335, 593–596 (2012).
Thresher, R. E., Koslow, J. A., Morison, A. K. & Smith, D. C. Depth-mediated reversal of the effects of climate change on long-term growth rates of exploited marine fish. Proc. Natl Acad. Sci. USA 104, 7461–7465 (2007).
Black, B. A. Climate-driven synchrony across tree, bivalve, and rockfish growth-increment chronologies of the northeast Pacific. Mar. Ecol. Prog. Ser. 378, 37–46 (2009).
Francis, R. I. C. C. & Horn, P. L. Transition zone in otoliths of orange roughy (Hoplostethus atlanticus) and its relationship to the onset of maturity. Mar. Biol. 129, 681–687 (1997).
Hale, R. & Swearer, S. E. Otolith microstructural and microchemical changes associated with settlement in the diadromous fish Galaxias maculatus. Mar. Ecol. Prog. Ser. 354, 229–234 (2008).
Jorgensen, T. Long-term changes in age at sexual maturity of northeast Arctic cod (Gadus morhua L.). ICES J. Mar. Sci. 46, 235–248 (1990).
Barbee, N. C. et al. Large-scale variation in life history traits of the widespread diadromous fish, Galaxias maculatus, reflects geographic differences in local environmental conditions. Mar. Freshwat. Res. 62, 790–800 (2011).
Chan, K. S., Zhang, T. Y. & Bailey, K. M. Otolith biochronology reveals factors underlying dynamics in marine fish larvae. Mar. Ecol. Prog. Ser. 412, 1–10 (2010).
Sinclair, A. F., Swain, D. P. & Hanson, J. M. Measuring changes in the direction and magnitude of size-selective mortality in a commercial fish population. Can. J. Fish. Aquat. Sci. 59, 361–371 (2002).
Campana, S. E. Chemistry and composition of fish otoliths: Pathways, mechanisms and applications. Mar. Ecol. Prog. Ser. 188, 263–297 (1999).
Radtke, R. L., Showers, W., Moksness, E. & Lenz, P. Environmental information stored in otoliths: Insights from stable isotopes. Mar. Biol. 127, 161–170 (1996).
Lowenstam, H. A. & Weiner, S. On Biomineralization (Oxford Univ. Press, 1989).
Kalish, J. M. Otolith microchemistry: Validation of the effects of physiology, age and environment on otolith composition. J. Exp. Mar. Biol. Ecol. 132, 151–178 (1989).
Jones, J. B. & Campana, S. E. Stable oxygen isotope reconstruction of ambient temperature during the collapse of a cod (Gadus morhua) fishery. Ecol. Appl. 19, 1500–1514 (2009).
Gillanders, B. M. Otolith chemistry to determine movements of diadromous and freshwater fish. Aquat. Living Resour. 18, 291–300 (2005).
Crook, D. A., Macdonald, J. I. & Raadik, T. A. Evidence of diadromous movements in a coastal population of southern smelts (Retropinninae: Retropinna) from Victoria, Australia. Mar. Freshwat. Res. 59, 638–646 (2008).
Hoie, H., Folkvord, A. & Otterlei, E. Effect of somatic and otolith growth rate on stable isotopic composition of early juvenile cod (Gadus morhua L.) otoliths. J. Exp. Mar. Biol. Ecol. 289, 41–58 (2003).
Cuveliers, E. L., Bolle, L. J., Volckaert, F. A. M. & Maes, G. E. Influence of DNA isolation from historical otoliths on nuclear-mitochondrial marker amplification and age determination in an overexploited fish, the common sole (Solea solea L.). Mol. Ecol. Resour. 9, 725–732 (2009).
Nielsen, E. E. & Hansen, M. M. Waking the dead: The value of population genetic analyses of historical samples. Fish. Fish. 9, 450–461 (2008).
Morison, A. K., Robertson, S. G. & Smith, D. C. An integrated system for production fish aging: Image analysis and quality assurance. North Am. J. Fish Manage. 18, 587–598 (1998).
Black, B. A., Boehlert, G. W. & Yoklavich, M. M. Using tree-ring crossdating techniques to validate annual growth increments in long-lived fishes. Can. J. Fish. Aquat. Sci. 62, 2277–2284 (2005).
Helama, S., Schone, B. R., Black, B. A. & Dunca, E. Constructing long-term proxy series for aquatic environments with absolute dating control using a sclerochronological approach: Introduction and advanced applications. Mar. Freshwat. Res. 57, 591–599 (2006).
Campana, S. E. & Neilson, J. D. Microstructure of fish otoliths. Can. J. Fish. Aquat. Sci. 42, 1014–1032 (1985).
Punt, A. E., Walker, T. I., Taylor, B. L. & Pribac, F. Standardization of catch and effort data in a spatially-structured shark fishery. Fish Res. 45, 129–145 (2000).
Branch, T. A. et al. Fleet dynamics and fishermen behavior: Lessons for fisheries managers. Can. J. Fish. Aquat. Sci. 63, 1647–1668 (2006).
Ricker, W. E. Effects of size-selective mortality and sampling bias on estimates of growth, mortality, production and yield. J. Fish. Res. Board Can. 26, 479–541 (1969).
Francis, R. I. C. C. Back-calculation of fish length: A critical review. J. Fish Biol. 36, 883–904 (1990).
Wootton, R. J. Ecology of Teleost Fishes (Kluwer Academic, 1998).
Newman, S. J., Williams, D. M. & Russ, G. R. Age validation, growth and mortality rates of the tropical snappers (Pisces: Lutjanidae) Lutjanus adetii (Castelnau, 1873) and L. quinquelineatus (Bloch, 1790) from the central Great Barrier Reef, Australia. Mar. Freshwat. Res. 47, 575–584 (1996).
Morrissey, M. B. Exploiting natural history variation: Looking to fishes for quantitative genetic models of natural populations. Ecol. Freshwat. Fish 20, 328–345 (2011).
Weisberg, S., Spangler, G. & Richmond, L. S. Mixed effects models for fish growth. Can. J. Fish. Aquat. Sci. 67, 269–277 (2010).
Nussey, D. H., Wilson, A. J. & Brommer, J. E. The evolutionary ecology of individual phenotypic plasticity in wild populations. J. Evol. Biol. 20, 831–844 (2007).
Nussey, D. H., Clutton-Brock, T. H., Elston, D. A., Albon, S. D. & Kruuk, L. E. B. Phenotypic plasticity in a maternal trait in red deer. J. Anim. Ecol. 74, 387–396 (2005).
Kearney, M. & Porter, W. Mechanistic niche modelling: Combining physiological and spatial data to predict species' ranges. Ecol. Lett. 12, 334–350 (2009).
Harley, C. D. G. Climate change, keystone predation, and biodiversity loss. Science 334, 1124–1127 (2011).
Cook, E. R., Briffa, K. R., Meko, D. M., Graybill, D. A. & Funkhouser, G. The segment length curse in long tree-ring chronology development for palaeoclimatic studies. Holocene 5, 229–237 (1995).
McMahon, S. M. et al. Improving assessment and modelling of climate change impacts on global terrestrial biodiversity. Trends Ecol. Evol. 26, 249–259 (2011).
Anchukaitis, K. J. et al. Forward modeling of regional scale tree-ring patterns in the southeastern United States and the recent influence of summer drought. Geophys. Res. Lett. 33, L04705 (2006).
Hoeke, R. K., Jokiel, P. L., Buddemeier, R. W. & Brainard, R. E. Projected changes to growth and mortality of Hawaiian corals over the next 100 years. PLoS One 6, e18038 (2011).
Buckley, L. B. et al. Can mechanism inform species' distribution models? Ecol. Lett. 13, 1041–1054 (2010).
Harley, C. D. G. et al. The impacts of climate change in coastal marine systems. Ecol. Lett. 9, 228–241 (2006).
Chen, P. Y., Welsh, C. & Hamann, A. Geographic variation in growth response of Douglas fir to interannual climate variability and projected climate change. Glob. Change Biol. 16, 3374–3385 (2010).
Matta, M. E., Black, B. A. & Wilderbuer, T. K. Climate-driven synchrony in otolith growth-increment chronologies for three Bering Sea flatfish species. Mar. Ecol. Prog. Ser. 413, 137–145 (2010).
Rypel, A. L., Haag, W. R. & Findlay, R. H. Pervasive hydrologic effects on freshwater mussels and riparian trees in southeastern floodplain systems. Wetlands 29, 497–504 (2009).
Stocks, J., Stewart, J., Gray, C. A. & West, R. J. Using otolith increment widths to infer spatial, temporal and gender variation in the growth of sand whiting Sillago ciliata. Fisheries Manage. Ecol. 18, 121–131 (2011).
Pereira, D. L., Bingham, C., Spangler, G. R., Conner, D. J. & Cunningham, P. K. in Recent Developments in Fish Otolith Research (eds Secor, D. H., Dean, J. M. & Campana, S. E.) 177–196 (Univ. South Carolina Press, 1995).
LeBreton, G. T. O. & Beamish, F. H. Interannual growth variation in fish and tree rings. Can. J. Fish. Aquat. Sci. 57, 2345–2356 (2000).
Weisberg, S. Using hard-part increment data to estimate age and environmental effects. Can. J. Fish. Aquat. Sci. 50, 1229–1237 (1993).
Walsh, C. T., Gray, C. A., West, R. J., van der Meulen, D. E. & Williams, L. F. G. Growth, episodic recruitment and age truncation in populations of a catadromous percichthyid, Macquaria colonorum. Mar. Freshwat. Res. 61, 397–407 (2010).
Sharpe, D. M. T. & Hendry, A. P. Life history change in commercially exploited fish stocks: An analysis of trends across studies. Evol. Appl. 2, 260–275 (2009).
Acknowledgements
We thank A. Hobday and G. Tuck for helpful comments on earlier versions of this manuscript. This project was funded by CSIRO's Wealth from Oceans Flagship and J.R.M. was supported by a CSIRO OCE Postdoctoral Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Morrongiello, J., Thresher, R. & Smith, D. Aquatic biochronologies and climate change. Nature Clim Change 2, 849–857 (2012). https://doi.org/10.1038/nclimate1616
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nclimate1616
This article is cited by
-
Comparative demography of surgeonfishes from the tropical western Pacific
Reviews in Fish Biology and Fisheries (2024)
-
Otolith biochronology for the long-term reconstruction of growth and stock dynamics of fish
Reviews in Fish Biology and Fisheries (2024)
-
Quantifying fish otolith mineralogy for trace-element chemistry studies
Scientific Reports (2022)
-
Inter-Annual Variabilities of the Body Weights of Two Cephalopod Species in the Yellow Sea Under Different Environmental Conditions
Journal of Ocean University of China (2022)
-
Effects of increasing temperature and aestivation on biogenic amines, signal transduction pathways and metabolic enzyme activities in the sea cucumber (Apostichopus japonicus)
Marine Biology (2022)