Abstract
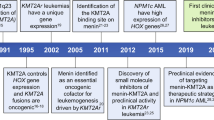
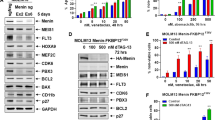
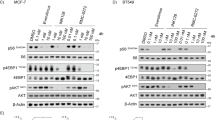
Translocations involving the mixed lineage leukemia (MLL) gene result in human acute leukemias with very poor prognosis. The leukemogenic activity of MLL fusion proteins is critically dependent on their direct interaction with menin, a product of the multiple endocrine neoplasia (MEN1) gene. Here we present what are to our knowledge the first small-molecule inhibitors of the menin–MLL fusion protein interaction that specifically bind menin with nanomolar affinities. These compounds effectively reverse MLL fusion protein–mediated leukemic transformation by downregulating the expression of target genes required for MLL fusion protein oncogenic activity. They also selectively block proliferation and induce both apoptosis and differentiation of leukemia cells harboring MLL translocations. Identification of these compounds provides a new tool for better understanding MLL-mediated leukemogenesis and represents a new approach for studying the role of menin as an oncogenic cofactor of MLL fusion proteins. Our findings also highlight a new therapeutic strategy for aggressive leukemias with MLL rearrangements.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Pui, C.H. et al. Outcome of treatment in childhood acute lymphoblastic leukaemia with rearrangements of the 11q23 chromosomal region. Lancet 359, 1909–1915 (2002).
Liu, H., Cheng, E.H. & Hsieh, J.J. MLL fusions: pathways to leukemia. Cancer Biol. Ther. 8, 1204–1211 (2009).
Sorensen, P.H. et al. Molecular rearrangements of the MLL gene are present in most cases of infant acute myeloid leukemia and are strongly correlated with monocytic or myelomonocytic phenotypes. J. Clin. Invest. 93, 429–437 (1994).
Cox, M.C. et al. Chromosomal aberration of the 11q23 locus in acute leukemia and frequency of MLL gene translocation: results in 378 adult patients. Am. J. Clin. Pathol. 122, 298–306 (2004).
Hess, J.L. MLL: a histone methyltransferase disrupted in leukemia. Trends Mol. Med. 10, 500–507 (2004).
Popovic, R. & Zeleznik-Le, N.J. MLL: how complex does it get? J. Cell. Biochem. 95, 234–242 (2005).
Krivtsov, A.V. & Armstrong, S.A. MLL translocations, histone modifications and leukaemia stem-cell development. Nat. Rev. Cancer 7, 823–833 (2007).
Slany, R.K. The molecular biology of mixed lineage leukemia. Haematologica 94, 984–993 (2009).
Hess, J.L., Yu, B.D., Li, B., Hanson, R. & Korsmeyer, S.J. Defects in yolk sac hematopoiesis in Mll-null embryos. Blood 90, 1799–1806 (1997).
Lawrence, H.J. et al. Mice bearing a targeted interruption of the homeobox gene HOXA9 have defects in myeloid, erythroid and lymphoid hematopoiesis. Blood 89, 1922–1930 (1997).
Rozovskaia, T. et al. Upregulation of Meis1 and HoxA9 in acute lymphocytic leukemias with the t(4: 11) abnormality. Oncogene 20, 874–878 (2001).
Ayton, P.M. & Cleary, M.L. Transformation of myeloid progenitors by MLL oncoproteins is dependent on Hoxa7 and Hoxa9. Genes Dev. 17, 2298–2307 (2003).
Zeisig, B.B. et al. Hoxa9 and Meis1 are key targets for MLL-ENL–mediated cellular immortalization. Mol. Cell. Biol. 24, 617–628 (2004).
Wong, P., Iwasaki, M., Somervaille, T.C., So, C.W. & Cleary, M.L. Meis1 is an essential and rate-limiting regulator of MLL leukemia stem cell potential. Genes Dev. 21, 2762–2774 (2007); erratum 21, 3017 (2007).
Argiropoulos, B. & Humphries, R.K. Hox genes in hematopoiesis and leukemogenesis. Oncogene 26, 6766–6776 (2007).
Rice, K.L. & Licht, J.D. HOX deregulation in acute myeloid leukemia. J. Clin. Invest. 117, 865–868 (2007).
Ernst, P., Wang, J. & Korsmeyer, S.J. The role of MLL in hematopoiesis and leukemia. Curr. Opin. Hematol. 9, 282–287 (2002).
Slany, R.K. When epigenetics kills: MLL fusion proteins in leukemia. Hematol. Oncol. 23, 1–9 (2005).
Yokoyama, A. et al. The menin tumor suppressor protein is an essential oncogenic cofactor for MLL-associated leukemogenesis. Cell 123, 207–218 (2005).
Caslini, C. et al. Interaction of MLL amino terminal sequences with menin is required for transformation. Cancer Res. 67, 7275–7283 (2007).
Chen, Y.X. et al. The tumor suppressor menin regulates hematopoiesis and myeloid transformation by influencing Hox gene expression. Proc. Natl. Acad. Sci. USA 103, 1018–1023 (2006).
Chandrasekharappa, S.C. et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science 276, 404–407 (1997).
Marx, S.J. Molecular genetics of multiple endocrine neoplasia types 1 and 2. Nat. Rev. Cancer 5, 367–375 (2005).
Hughes, C.M. et al. Menin associates with a trithorax family histone methyltransferase complex and with the hoxc8 locus. Mol. Cell 13, 587–597 (2004).
Yokoyama, A. et al. Leukemia proto-oncoprotein MLL forms a SET1-like histone methyltransferase complex with menin to regulate Hox gene expression. Mol. Cell. Biol. 24, 5639–5649 (2004).
Grembecka, J., Belcher, A.M., Hartley, T. & Cierpicki, T. Molecular basis of the mixed lineage leukemia-menin interaction: implications for targeting mixed lineage leukemias. J. Biol. Chem. 285, 40690–40698 (2010).
Mayer, M. & Meyer, B. Group epitope mapping by saturation transfer difference NMR to identify segments of a ligand in direct contact with a protein receptor. J. Am. Chem. Soc. 123, 6108–6117 (2001).
Murai, M.J., Chruszcz, M., Reddy, G., Grembecka, J. & Cierpicki, T. Crystal structure of menin reveals binding site for mixed lineage leukemia (MLL) protein. J. Biol. Chem. 286, 31742–31748 (2011).
Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65, 55–63 (1983).
Lavau, C., Szilvassy, S.J., Slany, R. & Cleary, M.L. Immortalization and leukemic transformation of a myelomonocytic precursor by retrovirally transduced HRX-ENL. EMBO J. 16, 4226–4237 (1997).
Thiel, A.T. et al. MLL-AF9–induced leukemogenesis requires coexpression of the wild-type Mll allele. Cancer Cell 17, 148–159 (2010).
Drabkin, H.A. et al. Quantitative HOX expression in chromosomally defined subsets of acute myelogenous leukemia. Leukemia 16, 186–195 (2002).
Yokoyama, A., Lin, M., Naresh, A., Kitabayashi, I. & Cleary, M.L. A higher-order complex containing AF4 and ENL family proteins with P-TEFb facilitates oncogenic and physiologic MLL-dependent transcription. Cancer Cell 17, 198–212 (2010).
Thompson, A. et al. Global down-regulation of HOX gene expression in PML-RARα+ acute promyelocytic leukemia identified by small-array real-time PCR. Blood 101, 1558–1565 (2003).
Fiskus, W. et al. Histone deacetylase inhibitors deplete enhancer of zeste 2 and associated polycomb repressive complex 2 proteins in human acute leukemia cells. Mol. Cancer Ther. 5, 3096–3104 (2006).
Golub, T.R. et al. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science 286, 531–537 (1999).
Quentmeier, H. et al. Expression of HOX genes in acute leukemia cell lines with and without MLL translocations. Leuk. Lymphoma 45, 567–574 (2004).
Thomas, M. et al. Targeting MLL-AF4 with short interfering RNAs inhibits clonogenicity and engraftment of t(4;11)-positive human leukemic cells. Blood 106, 3559–3566 (2005).
Armstrong, S.A. et al. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat. Genet. 30, 41–47 (2002).
Milne, T.A. et al. Menin and MLL cooperatively regulate expression of cyclin-dependent kinase inhibitors. Proc. Natl. Acad. Sci. USA 102, 749–754 (2005).
Muntean, A.G. et al. The PAF complex synergizes with MLL fusion proteins at HOX loci to promote leukemogenesis. Cancer Cell 17, 609–621 (2010).
Milne, T.A. et al. MLL associates specifically with a subset of transcriptionally active target genes. Proc. Natl. Acad. Sci. USA 102, 14765–14770 (2005).
Acknowledgements
This work was supported by grants from the Leukemia and Lymphoma Society (Translational Research Program grant 6070-09 to J.G. and Leukemia and Lymphoma Society Specialized Center of Research grant to J.L.H.), US National Institutes of Health R01 (1R01 CA-160467-01 to J.G.), Cancer Center (University of Virginia to J.G. and T.C.), Children's Leukemia Research Association (to J.G.), American Cancer Society (RSG-11-082-01-DMC to T.C.) and startup funds to J.G. and T.C. provided by the Department of Pathology (University of Michigan). The authors greatly appreciate support from J. Bushweller (University of Virginia) for providing the research environment for carrying out part of these studies. We are grateful to M. Larsen from the Center for Chemical Genomics, University of Michigan, for technical expertise during HTS. We would like to thank J. Tan (University of Michigan) for discussion of experimental procedures and R. Craig from the Department of Pathology (University of Michigan) for technical support with flow cytometry experiments.
Author information
Authors and Affiliations
Contributions
J.G. and T.C. initiated the project, led the project team, designed experiments and analyzed results. S.H., T.P. and A.G.M. performed cellular assays. A.S., R.J.S. and H.D.S. synthesized compounds. M.J.M. and T.H. expressed and purified proteins and ran ITC and thermal shift assays. A.M.B. performed biochemical assays. J.L.H. designed experiments and analyzed results. J.G. and T.C. wrote the paper with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods, Supplementary Results, Supplementary Tables 1–4 and Supplementary Figures 1–14 (PDF 2471 kb)
Rights and permissions
About this article
Cite this article
Grembecka, J., He, S., Shi, A. et al. Menin-MLL inhibitors reverse oncogenic activity of MLL fusion proteins in leukemia. Nat Chem Biol 8, 277–284 (2012). https://doi.org/10.1038/nchembio.773
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.773
This article is cited by
-
Narazaciclib, a novel multi-kinase inhibitor with potent activity against CSF1R, FLT3 and CDK6, shows strong anti-AML activity in defined preclinical models
Scientific Reports (2024)
-
Identification of FDA-approved drugs that induce heart regeneration in mammals
Nature Cardiovascular Research (2024)
-
Inhibition of the protein menin shows early promise in leukaemia
Nature (2023)
-
The menin inhibitor revumenib in KMT2A-rearranged or NPM1-mutant leukaemia
Nature (2023)
-
Combinatorial targeting of a chromatin complex comprising Dot1L, menin and the tyrosine kinase BAZ1B reveals a new therapeutic vulnerability of endocrine therapy-resistant breast cancer
Breast Cancer Research (2022)