Abstract
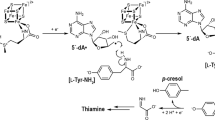
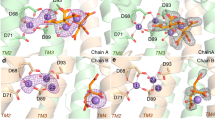
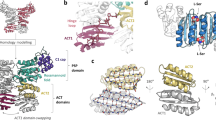
The Mycobacterium tuberculosis enzyme Rv2275 catalyzes the formation of cyclo(L-Tyr-L-Tyr) using two molecules of Tyr-tRNATyr as substrates. The three-dimensional (3D) structure of Rv2275 was determined to 2.0-Å resolution, revealing that Rv2275 is structurally related to the class Ic aminoacyl-tRNA synthetase family of enzymes. Mutagenesis and radioactive labeling suggests a covalent intermediate in which L-tyrosine is transferred from Tyr-tRNATyr to an active site serine (Ser88) by transesterification with Glu233 serving as a critical base, catalyzing dipeptide bond formation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Nishida, M., Mine, Y., Matsubara, T., Goto, S. & Kuwahara, S. J. Antibiot. (Tokyo) 25, 582–593 (1972).
Fukushima, K., Yazawa, K. & Arai, T. J. Antibiot. (Tokyo) 26, 175–176 (1973).
Ström, K., Sjogren, J., Broberg, A. & Schnurer, J. Appl. Environ. Microbiol. 68, 4322–4327 (2002).
Kanoh, K. et al. Biosci. Biotechnol. Biochem. 63, 1130–1133 (1999).
Balibar, C.J. & Walsh, C.T. Biochemistry 45, 15029–15038 (2006).
Gardiner, D.M., Cozijnsen, A.J., Wilson, L.M., Pedras, M.S. & Howlett, B.J. Mol. Microbiol. 53, 1307–1318 (2004).
Gardiner, D.M. & Howlett, B.J. FEMS Microbiol. Lett. 248, 241–248 (2005).
Schwarzer, D., Mootz, H.D. & Marahiel, M.A. Chem. Biol. 8, 997–1010 (2001).
Gondry, M. et al. Nat. Chem. Biol. 5, 414–420 (2009).
Belin, P. et al. Proc. Natl. Acad. Sci. USA 106, 7426–7431 (2009).
McLean, K.J. et al. J. Biol. Chem. 283, 33406–33416 (2008).
Krissinel, E. & Henrick, K. Acta Crystallogr. D Biol. Crystallogr. D60, 2256–2268 (2004).
Kobayashi, T. et al. Nat. Struct. Biol. 10, 425–432 (2003).
Hegde, S.S. & Blanchard, J.S. J. Biol. Chem. 278, 22861–22867 (2003).
Purdie, J.E. & Benoiton, N.L. J. Chem. Soc., Perkin Trans. 2 2, 1845–1852 (1973).
Steinberg, S.M. & Bada, J.L. J. Org. Chem. 48, 2295–2298 (1983).
Acknowledgements
We thank S. Del Amo and M. Callaway (Albert Einstein College of Medicine) for their help in cYY synthesis and ESI mass spectrometry, respectively. This work was supported by US National Institutes of Health Grant A133696.
Author information
Authors and Affiliations
Contributions
Funding was obtained by J.S.B. Experiments were conceived and designed by S.S.H., M.W.V. and J.S.B. S.S.H. cloned and purified Rv2275 and EcTyrRS, performed the kinetic analysis and did the radiolabeling experiments. M.W.V. determined the 3D structure of Rv2275 and cloned and purified the Rv2275 mutants. The manuscript was drafted by S.S.H. and M.W.V. and revised by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods, Supplementary Tables 1–3 and Supplementary Figures 1–7 (PDF 4543 kb)
Rights and permissions
About this article
Cite this article
Vetting, M., Hegde, S. & Blanchard, J. The structure and mechanism of the Mycobacterium tuberculosis cyclodityrosine synthetase. Nat Chem Biol 6, 797–799 (2010). https://doi.org/10.1038/nchembio.440
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.440
This article is cited by
-
Active site remodelling of a cyclodipeptide synthase redefines substrate scope
Communications Chemistry (2022)
-
Comparative genomics of Mycobacterium mucogenicum and Mycobacterium neoaurum clade members emphasizing tRNA and non-coding RNA
BMC Evolutionary Biology (2019)
-
Cyclization Reaction Catalyzed by Cyclodipeptide Synthases Relies on a Conserved Tyrosine Residue
Scientific Reports (2018)
-
Comparative genomic and functional analyses: unearthing the diversity and specificity of nematicidal factors in Pseudomonas putida strain 1A00316
Scientific Reports (2016)
-
Greatest hits
Nature Chemical Biology (2015)