Abstract
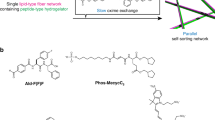
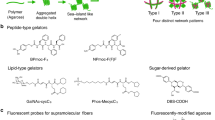
Self-sorted supramolecular nanofibres—a multicomponent system that consists of several types of fibre, each composed of distinct building units—play a crucial role in complex, well-organized systems with sophisticated functions, such as living cells. Designing and controlling self-sorting events in synthetic materials and understanding their structures and dynamics in detail are important elements in developing functional artificial systems. Here, we describe the in situ real-time imaging of self-sorted supramolecular nanofibre hydrogels consisting of a peptide gelator and an amphiphilic phosphate. The use of appropriate fluorescent probes enabled the visualization of self-sorted fibres entangled in two and three dimensions through confocal laser scanning microscopy and super-resolution imaging, with 80 nm resolution. In situ time-lapse imaging showed that the two types of fibre have different formation rates and that their respective physicochemical properties remain intact in the gel. Moreover, we directly visualized stochastic non-synchronous fibre formation and observed a cooperative mechanism.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Aida, T., Meijer, E. W. & Stupp, S. I. Functional supramolecular polymers. Science 335, 813–817 (2012).
He, Z., Jiang, W. & Schalley, C. A. Integrative self-sorting: a versatile strategy for the construction of complex supramolecular architecture. Chem. Soc. Rev. 44, 779–789 (2015).
Raeburn, J. & Adams, D. J. Multicomponent low molecular weight gelators. Chem. Commun. 51, 5170–5180 (2015).
Boekhoven, J., Koot, M., Wezendonk, T. A., Eelkema, R. & van Esch, J. H. A self-assembled delivery platform with post-production tunable release rate. J. Am. Chem. Soc. 134, 12908–12911 (2012).
Zhang, W. et al. Supramolecular linear heterojunction composed of graphite-like semiconducting nanotubular segments. Science 334, 340–343 (2011).
Alberts, B. et al. Molecular Biology of the Cell 5th edn, 965–1052 (Garland Science, 2008).
Omary, M. B., Coulombe, P. A. & McLean, W. H. I. Intermediate filament proteins and their associated diseases. N. Engl. J. Med. 351, 2087–2100 (2004).
Fuchs, E. & Cleveland, D. W. A structural scaffolding of intermediate filaments in health and disease. Science 279, 514–519 (1998).
Safont-Sempere, M. M., Fernández, G. & Würthner, F. Self-sorting phenomena in complex supramolecular systems. Chem. Rev. 111, 5784–5814 (2011).
Gao, Y., Shi, J., Yuan, D. & Xu, B. Imaging enzyme-triggered self-assembly of small molecules inside live cells. Nature Commun. 3, 1033 (2012).
Adler-Abramovich, L. & Gazit, E. The physical properties of supramolecular peptide assemblies: from building block association to technological applications. Chem. Soc. Rev. 43, 6881–6893 (2014).
Silva, G. A. et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science 303, 1352–1355 (2004).
Heeres, A. et al. Orthogonal self-assembly of low molecular weight hydrogelators and surfactants. J. Am. Chem. Soc. 125, 14252–14253 (2003).
Sugiyasu, K., Kawano, S.-i., Fujita, N. & Shinkai, S. Self-sorting organogels with p–n heterojunction points. Chem. Mater. 20, 2863–2865 (2008).
Ghosh, S., Li, X.-Q., Stepanenko, V. & Würthner, F. Control of H- and J-type π stacking by peripheral alkyl chains and self-sorting phenomena in perylene bisimide homo- and heteroaggregates. Chem. Eur. J. 14, 11343–11357 (2008).
Pal, A., Besenius, P. & Sijbesma, R. P. Self-sorting in rodlike micelles of chiral bisurea bolaamphiphiles. J. Am. Chem. Soc. 133, 12987–12989 (2011).
Prasanthkumar, S. et al. Organic donor–acceptor assemblies form coaxial p–n heterojunctions with high photoconductivity. Angew. Chem. Int. Ed. 54, 946–950 (2015).
Ikeda, M. et al. Montmorillonite—supramolecular hydrogel hybrid for fluorocolorimetric sensing of polyamines. J. Am. Chem. Soc. 133, 1670–1673 (2011).
Colquhoun, C. et al. The effect of self-sorting and co-assembly on the mechanical properties of low molecular weight hydrogels. Nanoscale 6, 13719–13725 (2014).
Adhikari, B., Nanda, J. & Banerjee, A. Multicomponent hydrogels from enantiomeric amino acid derivatives: helical nanofibers, handedness and self-sorting. Soft Matter 7, 8913–8922 (2011).
Molla, M. R., Das, A. & Ghosh, S. Self-sorted assembly in a mixture of donor and acceptor chromophores. Chem. Eur. J. 16, 10084–10093 (2010).
Smith, M. M. & Smith, D. K. Self-sorting multi-gelator gels–mixing and ageing effects in thermally addressable supramolecular soft nanomaterials. Soft Matter 7, 4856–4860 (2011).
Abul-Haija, Y. M. et al. Biocatalytically triggered co-assembly of two-component core/shell nanofibers. Small 10, 973–979 (2014).
Yu, G., Yan, X., Han, C. & Huang, F. Characterization of supramolecular gels. Chem. Soc. Rev. 42, 6697–6722 (2013).
Görl, D., Zhang, X., Stepanenko, V. & Würthner, F. Supramolecular block copolymers by kinetically controlled co-self-assembly of planar and core-twisted perylene bisimides. Nature Commun. 6, 7009 (2015).
Draper, E. R., Eden, E. G. B., McDonald, T. O. & Adams, D. J. Spatially resolved multicomponent gels. Nature Chem. 7, 848–852 (2015).
Morris, K. L. et al. Chemically programmed self-sorting of gelator networks. Nature Commun. 4, 1480 (2013).
Smulders, M. M. J. et al. How to distinguish isodesmic from cooperative supramolecular polymerisation. Chem. Eur. J. 16, 362–367 (2010).
Boekhoven, J., Hendriksen, W. E., Koper, G. J. M., Eelkema, R. & van Esch, J. H. Transient assembly of active materials fueled by a chemical reaction. Science 349, 1075–1079 (2015).
Aliprandi, A., Mauro, M. & De Cola, L. Controlling and imaging biomimetic self-assembly. Nature Chem. 8, 10–15 (2016).
Albertazzi, L. et al. Probing exchange pathways in one-dimensional aggregates with super-resolution microscopy. Science 344, 491–495 (2014).
Baker, M. B. et al. Consequences of chirality on the dynamics of a water-soluble supramolecular polymer. Nature Commun. 6, 6234 (2015).
Liang, Y., Lynn, D. G. & Berland, K. M. Direct observation of nucleation and growth in amyloid self-assembly. J. Am. Chem. Soc. 132, 6306–6308 (2010).
Ikeda, M. et al. Installing logic-gate responses to a variety of biological substances in supramolecular hydrogel–enzyme hybrids. Nature Chem. 6, 511–518 (2014).
Komatsu, H. et al. Supramolecular hydrogel exhibiting four basic logic gate functions to fine-tune substance release. J. Am. Chem. Soc. 131, 5580–5585 (2009).
Komatsu, H., Tsukiji, S., Ikeda, M. & Hamachi, I. Stiff, multistimuli-responsive supramolecular hydrogels as unique molds for 2D/3D microarchitectures of live cells. Chem. Asian J. 6, 2368–2375 (2011).
Hirst, A. R., Huang, B., Castelletto, V., Hamley, I. W. & Smith, D. K. Self-organisation in the assembly of gels from mixtures of different dendritic peptide building blocks. Chem. Eur. J. 13, 2180–2188 (2007).
Kiyonaka, S., Sugiyasu, K., Shinkai, S. & Hamachi, I. First thermally responsive supramolecular polymer based on glycosylated amino acid. J. Am. Chem. Soc. 124, 10954–10955 (2002).
Ikeda, M., Tanida, T., Yoshii, T. & Hamachi, I. Rational molecular design of stimulus-responsive supramolecular hydrogels based on dipeptides. Adv. Mater. 23, 2819–2822 (2011).
Kiyonaka, S. et al. Semi-wet peptide/protein array using supramolecular hydrogel. Nature Mater. 3, 58–64 (2004).
Comeau, J. W. D., Costantino, S. & Wiseman, P. W. A guide to accurate fluorescence microscopy colocalization measurements. Biophys. J. 91, 4611–4622 (2006).
Dunn, K. W., Kamocka, M. M. & McDonald, J. H. A practical guide to evaluating colocalization in biological microscopy. Am. J. Physiol. Cell Physiol. 300, C723–C742 (2011).
Adler, J. & Parmryd, I. Quantifying colocalization by correlation: the Pearson correlation coefficient is superior to the Mander's overlap coefficient. Cytom. Part A 77A, 733–742 (2010).
Hell, S. W. & Wichmann, J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 19, 780–782 (1994).
Tamaru, S.-i. et al. Fluidic supramolecular nano- and microfibres as molecular rails for regulated movement of nanosubstances. Nature Commun. 1, 20 (2010).
Thompson, N. L., Burghardt, T. P. & Axelrod, D. Measuring surface dynamics of biomolecules by total internal reflection fluorescence with photobleaching recovery or correlation spectroscopy. Biophys. J. 33, 435–454 (1981).
Jayaraman, K. et al. Observing capillarity in hydrophobic silica nanotubes. J. Am. Chem. Soc. 127, 17385–17392 (2005).
Acknowledgements
The authors thank K. Matsuda, M. Suginome and Y. Nagata (Kyoto University) for CD spectra measurements, U. Schwarz (Leica Microsystems) for STED microscopy, Y. Sato (Carl Zeiss Microimaging Co.) for making 3D CLSM images and T. Hirose (Kyoto University) for support with TEM. The authors acknowledge financial support from the CREST (Core Research for Evolutionary Science and Technology) programme of JST (the Japan Science and Technology Agency).
Author information
Authors and Affiliations
Contributions
M.I. and I.H. conceived the project. S.O. and T.T. synthesized and characterized the compounds. S.O. and H.S. measured and analysed the CD spectra. S.O., H.S. and T.Y. obtained the TEM, CLSM and STED images. H.S. conducted FRAP experiments. S.O. and H.S. performed in situ real-time imaging of fibre formation and degradation. The manuscript was written by S.O., H.S., R.K. and I.H. and edited by all co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 8435 kb)
Supplementary information
Supplementary movie 1 (AVI 3005 kb)
Supplementary information
Supplementary movie 2 (AVI 3416 kb)
Supplementary information
Supplementary movie 3 (AVI 4968 kb)
Supplementary information
Supplementary movie 4 (AVI 1771 kb)
Supplementary information
Supplementary movie 5 (AVI 1455 kb)
Supplementary information
Supplementary movie 6 (AVI 5305 kb)
Supplementary information
Supplementary movie 7 (AVI 3481 kb)
Supplementary information
SSupplementary movie 8 (AVI 1575 kb)
Rights and permissions
About this article
Cite this article
Onogi, S., Shigemitsu, H., Yoshii, T. et al. In situ real-time imaging of self-sorted supramolecular nanofibres. Nature Chem 8, 743–752 (2016). https://doi.org/10.1038/nchem.2526
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2526
This article is cited by
-
Four distinct network patterns of supramolecular/polymer composite hydrogels controlled by formation kinetics and interfiber interactions
Nature Communications (2023)
-
Fluorescence microscopic visualization of functionalized hydrogels
NPG Asia Materials (2022)
-
Supramolecular systems chemistry through advanced analytical techniques
Analytical and Bioanalytical Chemistry (2022)
-
Self-sorting double network hydrogels with photo-definable biochemical cues as artificial synthetic extracellular matrix
Nano Research (2022)
-
Fluorescent amino acids as versatile building blocks for chemical biology
Nature Reviews Chemistry (2020)