Abstract
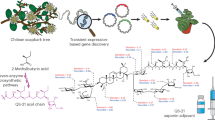
Adjuvants are materials added to vaccines to enhance the immunological response to an antigen. QS-21 is a natural product adjuvant under investigation in numerous vaccine clinical trials, but its use is constrained by scarcity, toxicity, instability and an enigmatic molecular mechanism of action. Herein we describe the development of a minimal QS-21 analogue that decouples adjuvant activity from toxicity and provides a powerful platform for mechanistic investigations. We found that the entire branched trisaccharide domain of QS-21 is dispensable for adjuvant activity and that the C4-aldehyde substituent, previously proposed to bind covalently to an unknown cellular target, is also not required. Biodistribution studies revealed that active adjuvants were retained preferentially at the injection site and the nearest draining lymph nodes compared with the attenuated variants. Overall, these studies have yielded critical insights into saponin structure–function relationships, provided practical synthetic access to non-toxic adjuvants, and established a platform for detailed mechanistic studies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Moyle, P. M. & Toth, I. Modern subunit vaccines: development, components, and research opportunities. ChemMedChem 8, 360–376 (2013).
Leroux-Roels, G. Unmet needs in modern vaccinology: adjuvants to improve the immune response. Vaccine 28, C25–C36 (2010).
Reed, S. G. et al. New horizons in adjuvants for vaccine development. Trends Immunol. 30, 23–32 (2009).
Kensil, C. R., Patel, U., Lennick, M. & Marciani, D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J. Immunol. 146, 431–437 (1991).
Kim, S. K. et al. Comparison of the effect of different immunological adjuvants on the antibody and T-cell response to immunization with MUC1–KLH and GD3–KLH conjugate cancer vaccines. Vaccine 18, 597–603 (1999).
Soltysik, S., Bedore, D. A. & Kensil, C. R. Adjuvant activity of QS-21 isomers. Ann. NY Acad. Sci. 690, 392–395 (1993).
Jacobsen, N. E. et al. Structure of the saponin adjuvant QS-21 and its base-catalyzed isomerization product by 1H and natural abundance 13C NMR spectroscopy. Carbohydr. Res. 280, 1–14 (1996).
Ragupathi, G., Gardner, J. R., Livingston, P. O. & Gin, D. Y. Natural and synthetic saponin adjuvant QS-21 for vaccines against cancer. Expert Rev. Vaccines 10, 463–470 (2011).
Agnandji, S. T. et al. First results of Phase 3 trial of RTS,S/AS01 malaria vaccine in African children. New Engl. J. Med. 365, 1863–1875 (2011).
Kennedy, J. S. et al. The safety and tolerability of an HIV-1 DNA prime-protein boost vaccine (DP6-001) in healthy adult volunteers. Vaccine 26, 4420–4424 (2008).
Vandepapeliere, P. et al. Vaccine adjuvant systems containing monophosphoryl lipid A and QS21 induce strong and persistent humoral and T cell responses against hepatitis B surface antigen in healthy adult volunteers. Vaccine 26, 1375–1386 (2008).
Von Eschen, K. et al. The candidate tuberculosis vaccine Mtb72F/AS02A: tolerability and immunogenicity in humans. Hum. Vaccin. 5, 475–482 (2009).
Vellas, B. et al. Long-term follow-up of patients immunized with AN1792: reduced functional decline in antibody responders. Curr. Alzheimer Res. 6, 144–151 (2009).
Wang, P., Kim, Y. J., Navarro-Villalobos, M., Rohde, B. D. & Gin, D. Y. Synthesis of the potent immunostimulatory adjuvant QS-21A. J. Am. Chem. Soc. 127, 3256–3257 (2005).
Deng, K. et al. Synthesis of QS-21-xylose: establishment of the immunopotentiating activity of synthetic QS-21 adjuvant with a melanoma vaccine. Angew. Chem. Int. Ed. 47, 6395–6398 (2008).
Adams, M. M. et al. Design and synthesis of potent Quillaja saponin vaccine adjuvants. J. Am. Chem. Soc. 132, 1939–1945 (2010).
Chea, E. K. et al. Synthesis and preclinical evaluation of QS-21 variants leading to simplified vaccine adjuvants and mechanistic probes. J. Am. Chem. Soc. 134, 13448–13457 (2012).
Soltysik, S. et al. Structure/function studies of QS-21 adjuvant: assessment of triterpene aldehyde and glucuronic acid roles in adjuvant function. Vaccine 13, 1403–1410 (1995).
Seevers, R. H. & Counsell, R. E. Radioiodination techniques for small organic molecules. Chem. Rev. 82, 575–590 (1982).
Duewell, P. et al. ISCOMATRIX adjuvant combines immune activation with antigen delivery to dendritic cells in vivo leading to effective cross-priming of CD8+ T cells. J. Immunol. 187, 55–63 (2011).
Scott, M. T., Goss-Sampson, M. & Bomford, R. Adjuvant activity of saponin: antigen localization studies. Int. Arch. Allergy Appl. Immunol. 77, 409–412 (1985).
Elliott, D. F. & Kon, G. A. R. Sapogenins. VI. Quillaic acid. J. Chem. Soc. 1130–1135 (1939).
Nico, D., Santos, F. N., Borja-Cabrera, G. P., Palatnik, M. & Palatnik de Sousa, C. B. Assessment of the monoterpene, glycidic and triterpene-moieties' contributions to the adjuvant function of the CP05 saponin of Calliandra pulcherrima Benth during vaccination against experimental visceral leishmaniasis. Vaccine 25, 649–658 (2007).
Castro-Diaz, N. et al. Saponins from the Spanish saffron Crocus sativus are efficient adjuvants for protein-based vaccines. Vaccine 30, 388–397 (2012).
Oda, K. et al. Adjuvant and haemolytic activities of 47 saponins derived from medicinal and food plants. Biol. Chem. 381, 67–74 (2000).
Pink, J. R. & Kieny, M. P. Fourth meeting on novel adjuvants currently in/close to human clinical testing. Vaccine 22, 2097–2102 (2004).
Kensil, C. R. Saponins as vaccine adjuvants. Crit. Rev. Ther. Drug Carrier Syst. 13, 1–55 (1996).
Marciani, D. J. Vaccine adjuvants: role and mechanisms of action in vaccine immunogenicity. Drug Discov. Today 8, 934–943 (2003).
Nimmerjahn, F. & Ravetch, J. V. Divergent immunoglobulin G subclass activity through selective Fc receptor binding. Science 310, 1510–1512 (2005).
Kensil, C. R. & Kammer, R. QS-21: a water-soluble triterpene glycoside adjuvant. Exp. Opin. Invest. Drugs 7, 1475–1482 (1998).
Bomford, R. Studies on the cellular site of action of the adjuvant activity of saponin for sheep erythrocytes. Int. Arch. Allergy Appl. Immunol. 67, 127–131 (1982).
Calabro, S. et al. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine 29, 1812–1823 (2011).
Dupuis, M., McDonald, D. M. & Ott, G. Distribution of adjuvant MF59 and antigen gD2 after intramuscular injection in mice. Vaccine 18, 434–439 (1999).
Dupuis, M. et al. Dendritic cells internalize vaccine adjuvant after intramuscular injection. Cell. Immunol. 186, 18–27 (1998).
Kim, S-K., Ragupathi, G., Cappello, S., Kagan, E. & Livingston, P. O. Effect of immunological adjuvant combinations on the antibody and T-cell response to vaccination with MUC1–KLH and GD3–KLH conjugates. Vaccine 19, 530–537 (2000).
Acknowledgements
Dedicated to the memory of our mentor and colleague, David Y. Gin (1967–2011). We thank S. J. Danishefsky, M. M. Adams and W. E. Walkowicz for helpful discussions, G. Sukenick, H. Liu, H. Fang and S. Rusli for expert NMR and mass spectral support and K. K. Sevak, N. Ramos and C. B. Davis for technical support with biodistribution and radiometric studies. Generous financial support was provided by the European Commission (Marie Curie International Outgoing Fellowship to A.F-T.), the National Institutes of Health (R01 AI085622 to J.S.L. and D.Y.G., R01 GM058833 to D.S.T. and D.Y.G., CCSG P30 CA008748 in support of the Center of Comparative Medicine and Pathology and the Radiochemistry and Molecular Imaging Probe Core), William and Alice Goodwin and the Commonwealth Foundation for Cancer Research and the Experimental Therapeutics Center of MSKCC.
Author information
Authors and Affiliations
Contributions
A.F-T., E.K.C., N.P., J.R.G., G.R., J.S.L., D.S.T. and D.Y.G. conceived and designed the experiments. A.F-T. and E.K.C. performed the syntheses. C.G. performed the preclinical mouse-vaccination experiments. C.G. and N.P. performed the biodistribution experiments. J.R.G. performed the fluorescence imaging experiments. A.F-T., E.K.C., N.P., J.R.G., P.O.L., G.R., J.S.L., D.S.T. and D.Y.G. analysed the data. A.F-T. and D.S.T. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.R.G., P.O.L., G.R. and D.Y.G are founders of Adjuvance Technologies Inc. and have financial interests in the company.
Supplementary information
Supplementary information
Supplementary information (PDF 24985 kb)
Rights and permissions
About this article
Cite this article
Fernández-Tejada, A., Chea, E., George, C. et al. Development of a minimal saponin vaccine adjuvant based on QS-21. Nature Chem 6, 635–643 (2014). https://doi.org/10.1038/nchem.1963
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1963
This article is cited by
-
Disease-Modifying Therapies for Alzheimer's Disease: More Questions than Answers
Neurotherapeutics (2022)
-
Natural and synthetic carbohydrate-based vaccine adjuvants and their mechanisms of action
Nature Reviews Chemistry (2021)
-
Plasmodium falciparum pre-erythrocytic stage vaccine development
Malaria Journal (2020)
-
Immunological evaluation of two novel engineered Plasmodium vivax circumsporozoite proteins formulated with different human-compatible vaccine adjuvants in C57BL/6 mice
Medical Microbiology and Immunology (2019)
-
Potent response of QS-21 as a vaccine adjuvant in the skin when delivered with the Nanopatch, resulted in adjuvant dose sparing
Scientific Reports (2016)