Abstract
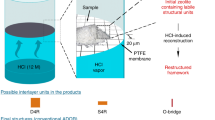
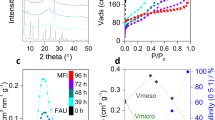
The properties of zeolites, and thus their suitability for different applications, are intimately connected with their structures. Synthesizing specific architectures is therefore important, but has remained challenging. Here we report a top-down strategy that involves the disassembly of a parent zeolite, UTL, and its reassembly into two zeolites with targeted topologies, IPC-2 and IPC-4. The three zeolites are closely related as they adopt the same layered structure, and they differ only in how the layers are connected. Choosing different linkers gives rise to different pore sizes, enabling the synthesis of materials with predetermined pore architectures. The structures of the resulting zeolites were characterized by interpreting the X-ray powder-diffraction patterns through models using computational methods; IPC-2 exhibits orthogonal 12- and ten-ring channels, and IPC-4 is a more complex zeolite that comprises orthogonal ten- and eight-ring channels. We describe how this method enables the preparation of functional materials and discuss its potential for targeting other new zeolites.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Breck, D. W. Zeolite Molecular Sieves (Krieger, 1984).
Barrer, R. M. Zeolites and their synthesis. Zeolites 1, 130–140 (1981).
Barrer, R. M. Hydrothermal Chemistry of Zeolites (Academic Press, 1982).
Čejka, J., van Bekkum, H., Corma, A. & Schüth, F. Introduction to Zeolite Science and Practice 3rd edn (Studies in Surface Science Catalysis Vol. 168, Elsevier, 2007).
Xu, R., Pang, W., Yu, J., Huo, Q. & Chen, J. Chemistry of Zeolites and Related Porous Materials (Wiley, 2007).
Jiang, J. et al. Synthesis and structure determination of the hierarchical meso-microporous zeolite ITQ-43. Science 333, 1131–1134 (2011).
Baerlocher, C., Weber, T., McCusker, L. B., Palatinus, L. & Zones, S. I. Unraveling the perplexing structure of the zeolite SSZ-57. Science 333, 1134–1137 (2011).
Simancas, R. et al. Modular organic structure-directing agents for the synthesis of zeolites. Science 330, 1219–1222 (2010).
Sun, J. et al. The ITQ-37 mesoporous chiral zeolite. Nature 458, 1154–U90 (2009).
Tang, L. et al. A zeolite family with chiral and achiral structures built from the same building layer. Nature Mater. 7, 381–385 (2008).
Baerlocher, C. et al. Ordered silicon vacancies in the framework structure of the zeolite catalyst SSZ-74. Nature Mater. 7, 631–635 (2008).
Baerlocher, C. et al. Structure of the polycrystalline zeolite catalyst IM-5 solved by enhanced charge flipping. Science 315, 1113–1116 (2007).
Paillaud, J. L., Harbuzaru, B., Patarin, J. & Bats, N. Extra-large-pore zeolites with two-dimensional channels formed by 14 and 12 rings. Science 304, 990–992 (2004).
Corma, A., Diaz-Cabanas, M. J., Rey, F., Nicolooulas, S. & Boulahya, K. ITQ-15: The first ultralarge pore zeolite with a bi-directional pore system formed by intersecting 14- and 12-ring channels, and its catalytic implications. Chem. Commun. 1356–1357 (2004).
Roth, W. J. et al. Postsynthesis transformation of three-dimensional framework into a lamellar zeolite with modifiable architecture. J. Am. Chem. Soc. 133, 6130–6133 (2011).
Lee, K., Murray, E. D., Kong, L. Z., Lundqvist, B. I. & Langreth, D. C. Higher-accuracy van der Waals density functional. Phys. Rev. B 82, 081101 (2010).
Kresse, G. & Hafner, J. Ab-initio molecular-dynamics for open-shell transition-metals. Phys. Rev. B 48, 13115–13118 (1993).
Cooper, E. R. et al. Ionic liquids and eutectic mixtures as solvent and template in synthesis of zeolite analogues. Nature 430, 1012–1016 (2004).
Wu, P. et al. Methodology for synthesizing crystalline metallosilicates with expanded pore windows through molecular alkoxysilylation of zeolitic lamellar precursors. J. Am. Chem. Soc. 130, 8178–8187 (2008).
Inagaki, S., Yokoi, T., Kubota, Y. & Tatsumi, T. Unique adsorption properties of organic–inorganic hybrid zeolite IEZ-1 with dimethylsilylene moieties. Chem. Commun. 5188–5190 (2007).
Gies, H. et al. Interlayer expansion of the hydrous layer silicate RUB-36 to a functionalized, microporous framework silicate: crystal structure analysis and physical and chemical characterization. Chem. Mater. 24, 1536–1545 (2012).
Xiao, F-S. et al. Interlayer-expanded microporous titanosilicate catalysts with functionalized hydroxyl groups. ChemCatChem 3, 1442–1446 (2011).
Gies, H. et al. Interlayer expansion of the layered zeolite precursor RUB-39: a universal method to synthesize functionalized microporous silicates. Chem. Mater. 23, 2545–2554 (2011).
Verheyen, E. et al. Design of zeolite by inverse sigma transformation. Nature Mater. 11, 1059–1064 (2012).
Shamzhy, M. V. et al. Synthesis of isomorphously substituted extra-large pore UTL zeolites. J. Mater. Chem. 22, 15793–15803 (2012).
Baerlocher, C., Maier W. M. & Olson, D. H. Atlas of Zeolite Framework Types 6th edn (Elsevier, 2007).
Corma, A., Rey, F., Valencia, S., Jorda, J. L. & Rius, J. A zeolite with interconnected 8-, 10- and 12-ring pores and its unique catalytic selectivity. Nature Mater. 2, 493–497 (2003).
Corma, A., Puche, M., Rey, F., Sankar, G. & Teat, S. J. A zeolite structure (ITQ-13) with three sets of medium-pore crossing channels formed by 9- and 10-rings. Angew. Chem. Int. Ed. 42, 1156–1159 (2003).
Corma, A. et al. A zeolitic structure (ITQ-34) with connected 9- and 10-ring channels obtained with phosphonium cations as structure directing agents. J. Am. Chem. Soc. 130, 16482–16483 (2008).
Castaneda, R., Corma, A., Fornes, V., Rey, F. & Rius, J. Synthesis of a new zeolite structure ITQ-24, with intersecting 10- and 12-membered ring pores. J. Am. Chem. Soc. 125, 7820–7821 (2003).
Dorset, D. L. et al. P-derived organic cations as structure-directing agents: synthesis of a high-silica zeolite (ITQ-27) with a two-dimensional 12-ring channel system. J. Am. Chem. Soc. 128, 8862–8867 (2006).
Marler, B. & Gies, H. Hydrous layer silicates as precursors for zeolites obtained through topotactic condensation: a review. Eur. J. Mineral. 24, 405–428 (2012).
Roth, W. J. & Čejka, J. Two-dimensional zeolites: dream or reality? Catal. Sci. Technol. 1, 43–53 (2011).
Shvets, O. V., Kasian, N., Zukal, A., Pinkas, J. & Čejka, J. The role of template structure and synergism between inorganic and organic structure directing agents in the synthesis of UTL zeolite. Chem. Mater. 22, 3482–3495 (2010).
Chlubna, P. et al. 3D to 2D routes to ultrathin and expanded zeolitic materials. Chem. Mater. 25, 542–547 (2013).
Acknowledgements
The authors acknowledge the Grant Agency of the Czech Republic (Center of Excellence – P106/12/G015). R.E.M. is a Royal Society Industry Fellow. We also thank the Leverhulme Trust and the Engineering and Physical Sciences Research Council (EP/K005499/1 and EP/K039210/1) for funding. J.Č. thanks Micromeritics for an Instrument Award.
Author information
Authors and Affiliations
Contributions
W.J.R., P.C. and O.S. completed the synthesis work, P.N., L.G. and M.P. the calculations, P.S.W. the Rietveld refinement, V.R.S. and S.E.A. the NMR spectroscopy and A.Z. the adsorption experiments. W.J.R., P.N., J.Č. and R.E.M. co-wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1406 kb)
Supplementary information
Atomic positions of experimental IPC-2 structure (CIF 5 kb)
Supplementary information
Atomic positions of experimental IPC-4 structure (CIF 5 kb)
Supplementary information
Calculated atomic positions for IPC-2 (equivalent to structure a in Figure S3) (CIF 5 kb)
Supplementary information
Calculated atomic positions for structure b in Figure S3 (CIF 5 kb)
Supplementary information
Calculated atomic positions for structure c in Figure S3 (CIF 9 kb)
Supplementary information
Calculated atomic positions for structure d in Figure S3 (CIF 5 kb)
Supplementary information
Calculated atomic positions for IPC-4 (equivalent to structure a in Figure S1) (CIF 7 kb)
Supplementary information
Calculated atomic positions for structure b in Figure S1 (CIF 4 kb)
Supplementary information
Calculated atomic positions for structure c in Figure S1 (CIF 7 kb)
Supplementary information
Calculated atomic positions for structure d in Figure S1 (CIF 4 kb)
Rights and permissions
About this article
Cite this article
Roth, W., Nachtigall, P., Morris, R. et al. A family of zeolites with controlled pore size prepared using a top-down method. Nature Chem 5, 628–633 (2013). https://doi.org/10.1038/nchem.1662
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1662
This article is cited by
-
Germanium-enriched double-four-membered-ring units inducing zeolite-confined subnanometric Pt clusters for efficient propane dehydrogenation
Nature Catalysis (2023)
-
Synthesis strategies and design principles for nanosized and hierarchical zeolites
Nature Synthesis (2022)
-
Theoretical design for zeolite synthesis
Science China Chemistry (2022)
-
Synthesis of Pure Silica Zeolites
Chemical Research in Chinese Universities (2022)
-
Interzeolite conversion of HY to hierarchical HZSM-5 catalyst via an ionothermal route and its excellent catalytic performance in methanol-to-aromatics reaction
Journal of Porous Materials (2022)