Abstract
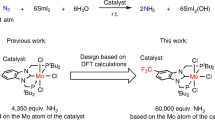
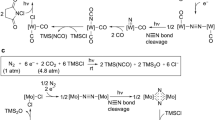
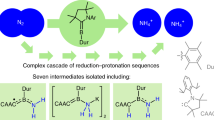
The synthesis of transition metal–dinitrogen complexes and the stoichiometric transformation of their coordinated dinitrogen into ammonia and hydrazine have been the subject of considerable research, with a view to achieving nitrogen fixation under ambient conditions. Since a single example in 2003, no examples have been reported of the catalytic conversion of dinitrogen into ammonia under ambient conditions. The dimolybdenum–dinitrogen complex bearing PNP pincer ligands was found to work as an effective catalyst for the formation of ammonia from dinitrogen, with 23 equiv. of ammonia being produced with the catalyst (12 equiv. of ammonia are produced based on the molybdenum atom of the catalyst). This is another successful example of the catalytic and direct conversion of dinitrogen into ammonia under ambient reaction conditions. We believe that the results described in this Article provide valuable information with which to develop a more effective nitrogen-fixation system under mild reaction conditions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ertl, G. In Catalytic Ammonia Synthesis (ed. Jennings, J. R.) (Plenum Press, 1991) and references therein.
Appl, M. Ammonia (Wiley-VCH, 1999) and references therein.
Einsle, O. et al. Nitrogenase MoFe-protein at 1.16 Å resolution: a central ligand in the FeMo-cofactor. Science 297, 1696–1700 (2002).
Dos Santos, P. C. et al. Substrate interactions with the nitrogenase active site. Acc. Chem. Res. 38, 208–214 (2005).
Dance, I. Elucidating the coordination chemistry and mechanism of biological nitrogen fixation. Chem. Asian J. 2, 936–946 (2007).
Kästner, J. & Blöchl, P. E. Ammonia production at the FeMo cofactor of nitrogenase: results from density functional theory. J. Am. Chem. Soc. 129, 2998–3006 (2007).
MacKay, B. A. & Fryzuk, M. D. Dinitrogen coordination chemistry: on the biomimetic borderland. Chem. Rev. 104, 385–401 (2004).
Gambarotta, S. & Scott, J. Multimetallic cooperative activation of N2 . Angew. Chem. Int. Ed. 43, 5298–5308 (2004).
Hidai, M. & Mizobe, Y. Research inspired by the chemistry of nitrogenase—novel metal complexes and their reactivity toward dinitrogen, nitriles, and alkynes. Can. J. Chem. 83, 358–374 (2005).
Himmel, H.-J. & Reiher, M. Intrinsic dinitrogen activation at bare metal atoms. Angew. Chem. Int. Ed. 45, 6264–6288 (2006).
Chirik, P. J. Dinitrogen functionalization with bis(cyclopentadienyl) complexes of zirconium and hafnium. Dalton Trans. 16–25 (2007).
Fryzuk, M. D. Side-on end-on bound dinitrogen: an activated bonding mode that facilitates functionalizing molecular nitrogen. Acc. Chem. Res. 42, 127–133 (2009).
Ballmann, J., Munhá, R. F. & Fryzuk, M. D. The hydride route to the preparation of dinitrogen complexes. Chem. Commun. 46, 1013–1025 (2010).
Bazhenova, T. A. & Shilov, A. E. Nitrogen-fixation in solution. Coord. Chem. Rev. 144, 69–145 (1995).
Shilov, A. E. Catalytic reduction of molecular nitrogen in solutions. Russ. Chem. Bull. 52, 2555–2562 (2003).
Shiina, K. Reductive silylation of molecular nitrogen via fixation to tris(trialkylsilyl)amine. J. Am. Chem. Soc. 94, 9266–9267 (1972).
Komori, K., Oshita, H., Mizobe, Y. & Hidai, M. Catalytic conversion of molecular nitrogen into silylamines using molybdenum and tungsten dinitrogen complexes. J. Am. Chem. Soc. 111, 1939–1940 (1989).
Komori, K., Sugiura, S., Mizobe, Y., Yamada, M. & Hidai, M. Syntheses and some reactions of trimethylsilylated dinitrogen complexes of tungsten and molybdenum. Bull. Chem. Soc. Jpn 62, 2953–2959 (1989).
Oshita, H., Mizobe, Y. & Hidai, M. Preparation and properties of molybdenum and tungsten dinitrogen complexes XLI: silylation and germylation of a coordinated dinitrogen in cis-[M(N2)2(PMe2Ph)4] (M=Mo, W) using R3ECl/NaI and R3ECl/Na mixtures (E=Si, Ge). X-ray structure of trans-[WI(NNGePh3)(PMe2Ph)4]·C6H6 . J. Organomet. Chem. 456, 213–220 (1993).
Mori, M. Activation of nitrogen for organic synthesis. J. Organomet. Chem. 689, 4210–4227 (2004) and references therein.
Yandulov, D. V. & Schrock, R. R. Catalytic reduction of dinitrogen to ammonia at a single molybdenum center. Science 301, 76–78 (2003).
Ritleng, V. et al. Molybdenum triamidoamine complexes that contain hexa-tert-butylterphenyl, hexamethylterphenyl, or p-bromohexaisopropylterphenyl substituents. An examination of some catalyst variations for the catalytic reduction of dinitrogen. J. Am. Chem. Soc. 126, 6150–6163 (2004).
Neese, F. The Yandulov/Schrock cycle and the nitrogenase reaction: pathways of nitrogen fixation studied by density functional theory. Angew. Chem. Int. Ed. 45, 196–199 (2006).
Schrock, R. R. Catalytic reduction of dinitrogen to ammonia at a single molybdenum center. Acc. Chem. Res. 38, 955–962 (2005).
Schrock, R. R. Catalytic reduction of dinitrogen to ammonia by molybdenum: theory versus experiment. Angew. Chem. Int. Ed. 47, 5512–5522 (2008).
Nishibayashi, Y., Iwai, S. & Hidai, M. Bimetallic system for nitrogen fixation: ruthenium-assisted protonation of coordinated N2 on tungsten with H2 . Science 279, 540–542 (1998).
Nishibayashi, Y., Takemoto, S., Iwai, S. & Hidai, M. Formation of ammonia in the reactions of a tungsten dinitrogen with ruthenium dihydrogen complexes under mild reaction conditions. Inorg. Chem. 39, 5946–5957 (2000).
Nishibayashi, Y., Iwai, S. & Hidai, M. A model for protonation of dinitrogen by nitrogenase: protonation of coordinated dinitrogen on tungsten with hydrosulfido-bridged dinuclear complexes. J. Am. Chem. Soc. 120, 10559–10560 (1998).
Nishibayashi, Y., Wakiji, I., Hirata, K., DuBois, M. R. & Hidai, M. Protonation of coordinated N2 on tungsten with H2 mediated by sulfido-bridged dinuclear molybdenum complexes. Inorg. Chem. 40, 578–580 (2001).
Nishibayashi, Y. et al. Buckminsterfullerenes: a non-metal system for nitrogen fixation. Nature 428, 279–280 (2004).
Yuki, M., Miyake, Y., Nishibayashi, Y., Wakiji, I. & Hidai, M. Synthesis and reactivity of tungsten– and molybdenum–dinitrogen complexes bearing ferrocenyldiphosphines toward protonolysis. Organometallics 27, 3947–3953 (2008).
Yuki, M., Midorikawa, T., Miyake, Y. & Nishibayashi, Y. Synthesis and protonolysis of tungsten– and molybdenum–dinitrogen complexes bearing ruthenocenyldiphosphines. Organometallics 28, 4741–4746 (2009).
Yuki, M., Miyake, Y. & Nishibayashi, Y. Preparation and protonation of tungsten– and molybdenum–dinitrogen complexes bearing bis(dialkylphosphinobenzene)chromiums as auxiliary ligands. Organometallics 28, 5821–5827 (2009).
van der Vlugt, J. I. & Reek, J. N. H. Neutral tridentate PNP ligands and their hybrid analogues: versatile non-innocent scaffolds for homogeneous catalysis. Angew. Chem. Int. Ed. 48, 8832–8846 (2009).
Chatt, J., Heath, G. A. & Richards, R. L. Diazene-N-(di-imide) and hydrazido-(2-)N-(aminoimido) complexes: the addition of acids to dinitrogen complexes. J. Chem. Soc. Dalton Trans. 2074–2082 (1974).
Hidai, M., Tominari, K. & Uchida, Y. Preparation and properties of dinitrogen–molybdenum complexes. J. Am. Chem. Soc. 94, 110–114 (1972).
Castellani, M. P., Geib, S. J., Rheingold, A. L. & Trogler, W. C. Syntheses, reactivities, molecular structures, and physical properties of paramagnetic bis(tetraphenylcyclopentadienyl) complexes of vanadium, chromium, cobalt, and nickel. Organometallics 6, 1703–1712 (1987).
Augustin-Nowacka, D. & Chmurzyñski, L. A potentiometric study of acid–base equilibria of substituted pyridines in acetonitrile. Anal. Chim. Acta 381, 215–220 (1999).
Nurminen, E. J., Mattinen, J. K. & Lönnberg, H. Nucleophilic and acid catalysis in phosphoramidite alcoholysis. J. Chem. Soc. Perkin Trans. 2, 2159–2165 (2001).
Fagnou, K. & Lautens, M. Halide effects in transition metal catalysis, Angew. Chem. Int. Ed. 41, 26–47 (2002).
Acknowledgements
The authors are grateful to Y.K. Kato and T. Shimada (University of Tokyo) for Raman spectroscopy measurements and to K. Nozaki and K. Nakano (University of Tokyo) for TOF MS analysis. This work was supported by Grant-in-Aids for Scientific Research for Young Scientists (S) (no. 19675002) and for Scientific Research on Priority Areas (no. 18066003) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Contributions
Y.N. directed and conceived the project and wrote the manuscript. K.A. designed and performed all experiments, including the X-ray study, and wrote the experimental details in the Supplementary Information. Y.M. carried out Raman spectroscopy measurements and TOF MS analysis. All authors discussed the results and commented on the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 983 kb)
Supplementary information
Crystallographic data for compound 1a (CIF 63 kb)
Supplementary information
Crystallographic data for compound 2a (CIF 31 kb)
Supplementary information
Crystallographic data for compound 3a (CIF 40 kb)
Supplementary information
Crystallographic data for compound 7a (CIF 43 kb)
Rights and permissions
About this article
Cite this article
Arashiba, K., Miyake, Y. & Nishibayashi, Y. A molybdenum complex bearing PNP-type pincer ligands leads to the catalytic reduction of dinitrogen into ammonia. Nature Chem 3, 120–125 (2011). https://doi.org/10.1038/nchem.906
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.906