Abstract
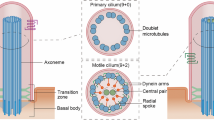
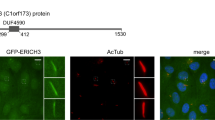
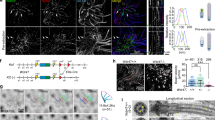
Cilia dysfunction has long been associated with cyst formation and ciliopathies1. More recently, misoriented cell division has been observed in cystic kidneys2, but the molecular mechanism leading to this abnormality remains unclear. Proteins of the intraflagellar transport (IFT) machinery are linked to cystogenesis and are required for cilia formation in non-cycling cells3,4. Several IFT proteins also localize to spindle poles in mitosis5,6,7,8, indicating uncharacterized functions for these proteins in dividing cells. Here, we show that IFT88 depletion induces mitotic defects in human cultured cells, in kidney cells from the IFT88 mouse mutant Tg737orpk and in zebrafish embryos. In mitosis, IFT88 is part of a dynein1-driven complex that transports peripheral microtubule clusters containing microtubule-nucleating proteins to spindle poles to ensure proper formation of astral microtubule arrays and thus proper spindle orientation. This work identifies a mitotic mechanism for a cilia protein in the orientation of cell division and has important implications for the etiology of ciliopathies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hildebrandt, F. & Otto, E. Cilia and centrosomes: a unifying pathogenic concept for cystic kidney disease?. Nat. Rev. Genet. 6, 928–940 (2005).
Fischer, E. et al. Defective planar cell polarity in polycystic kidney disease. Nat. Genet. 38, 21–23 (2006).
Pazour, G. J. et al. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J. Cell Biol. 151, 709–718 (2000).
Sun, Z. et al. A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development 131, 4085–4093 (2004).
Iomini, C., Tejada, K., Mo, W., Vaananen, H. & Piperno, G. Primary cilia of human endothelial cells disassemble under laminar shear stress. J. Cell Biol. 164, 811–817 (2004).
Robert, A. et al. The intraflagellar transport component IFT88/polaris is a centrosomal protein regulating G1-S transition in non-ciliated cells. J. Cell Sci. 120, 628–637 (2007).
Follit, J. A., Tuft, R. A., Fogarty, K. E. & Pazour, G. J. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol. Biol. Cell 17, 3781–3792 (2006).
Deane, J. A., Cole, D. G., Seeley, E. S., Diener, D. R. & Rosenbaum, J. L. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr. Biol. 11, 1586–1590 (2001).
Rieder, C. L., Faruki, S. & Khodjakov, A. The centrosome in vertebrates: more than a microtubule-organizing centre. Trends Cell Biol. 11, 413–419 (2001).
Scholey, J. M. Intraflagellar transport motors in cilia: moving along the cell’s antenna. J. Cell Biol. 180, 23–29 (2008).
Rosenbaum, J. L. & Witman, G. B. Intraflagellar transport. Nat. Rev. Mol. Cell Biol. 3, 813–825 (2002).
Pazour, G. J., Dickert, B. L. & Witman, G. B. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J. Cell Biol. 144, 473–481 (1999).
Luders, J. & Stearns, T. Microtubule-organizing centres: a re-evaluation. Nat. Rev. Mol. Cell Biol. 8, 161–167 (2007).
O’Connell, C. B. & Wang, Y. L. Mammalian spindle orientation and position respond to changes in cell shape in a dynein-dependent fashion. Mol. Biol. Cell 11, 1765–1774 (2000).
Toyoshima, F. & Nishida, E. Integrin-mediated adhesion orients the spindle parallel to the substratum in an EB1- and myosin X-dependent manner. EMBO J. 26, 1487–1498 (2007).
Murcia, N. S. et al. The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left-right axis determination. Development 127, 2347–2355 (2000).
Haycraft, C. J., Swoboda, P., Taulman, P. D., Thomas, J. H. & Yoder, B. K. The C. elegans homolog of the murine cystic kidney disease gene Tg737 functions in a ciliogenic pathway and is disrupted in osm-5 mutant worms. Development 128, 1493–1505 (2001).
Kramer-Zucker, A. G. et al. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer’s vesicle is required for normal organogenesis. Development 132, 1907–1921 (2005).
Han, Y. G., Kwok, B. H. & Kernan, M. J. Intraflagellar transport is required in Drosophila to differentiate sensory cilia but not sperm. Curr. Biol. 13, 1679–1686 (2003).
Tulu, U. S., Rusan, N. M. & Wadsworth, P. Peripheral, non-centrosome-associated microtubules contribute to spindle formation in centrosome-containing cells. Curr. Biol. 13, 1894–1899 (2003).
Rusan, N. M., Tulu, U. S., Fagerstrom, C. & Wadsworth, P. Reorganization of the microtubule array in prophase/prometaphase requires cytoplasmic dynein-dependent microtubule transport. J. Cell Biol. 158, 997–1003 (2002).
Hannak, E. et al. The kinetically dominant assembly pathway for centrosomal asters in Caenorhabditis elegans is γ-tubulin dependent. J. Cell Biol. 157, 591–602 (2002).
Rogers, S. L., Rogers, G. C., Sharp, D. J. & Vale, R. D. Drosophila EB1 is important for proper assembly, dynamics, and positioning of the mitotic spindle. J. Cell Biol. 158, 873–884 (2002).
Zimmerman, W. C., Sillibourne, J., Rosa, J. & Doxsey, S. J. Mitosis-specific anchoring of γ tubulin complexes by pericentrin controls spindle organization and mitotic entry. Mol. Biol. Cell 15, 3642–3657 (2004).
Green, R. A., Wollman, R. & Kaplan, K. B. APC and EB1 function together in mitosis to regulate spindle dynamics and chromosome alignment. Mol. Biol. Cell 16, 4609–4622 (2005).
Tulu, U. S., Fagerstrom, C., Ferenz, N. P. & Wadsworth, P. Molecular requirements for kinetochore-associated microtubule formation in mammalian cells. Curr. Biol. 16, 536–541 (2006).
Walczak, C. E., Vernos, I., Mitchison, T. J., Karsenti, E. & Heald, R. A model for the proposed roles of different microtubule-based motor proteins in establishing spindle bipolarity. Curr. Biol. 8, 903–913 (1998).
Gaglio, T., Dionne, M. A. & Compton, D. A. Mitotic spindle poles are organized by structural and motor proteins in addition to centrosomes. J. Cell Biol. 138, 1055–1066 (1997).
Echeverri, C. J., Paschal, B. M., Vaughan, K. T. & Vallee, R. B. Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J. Cell Biol. 132, 617–633 (1996).
Corthesy-Theulaz, I., Pauloin, A. & Pfeffer, S. R. Cytoplasmic dynein participates in the centrosomal localization of the Golgi complex. J. Cell Biol. 118, 1333–1345 (1992).
Vaisberg, E. A., Grissom, P. M. & McIntosh, J. R. Mammalian cells express three distinct dynein heavy chains that are localized to different cytoplasmic organelles. J. Cell Biol. 133, 831–842 (1996).
Young, A., Dictenberg, J. B., Purohit, A., Tuft, R. & Doxsey, S. J. Cytoplasmic dynein-mediated assembly of pericentrin and γ tubulin onto centrosomes. Mol. Biol. Cell 11, 2047–2056 (2000).
Yamashita, Y. M., Mahowald, A. P., Perlin, J. R. & Fuller, M. T. Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science 315, 518–521 (2007).
Wheatley, D. N. Primary cilia in normal and pathological tissues. Pathobiology 63, 222–238 (1995).
Finetti, F. et al. Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nat. Cell Biol. 11, 1332–1339 (2009).
Ross, A. J. et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat. Genet. 37, 1135–1140 (2005).
Pazour, G. J., San Agustin, J. T., Follit, J. A., Rosenbaum, J. L. & Witman, G. B. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr. Biol. 12, R378–R380 (2002).
Follit, J. A., Xu, F., Keady, B. T. & Pazour, G. J. Characterization of mouse IFT complex B. Cell Motil Cytoskeleton 66, 457–468 (2009).
Mikule, K. et al. Loss of centrosome integrity induces p38-p53-p21-dependent G1-S arrest. Nat. Cell Biol. 9, 160–170 (2007).
Westerfield, M. The Zebrafish book : a guide for the laboratory use of zebrafish (Brachydanio rerio) (Univ. Oregon Press, 1993).
Acknowledgements
We thank G. Pazour, G. Witman and P. Wadsworth for thoughtful discussions on this work, and S. Redick for assistance with microscopy. We are particularly grateful to L. Covassin-Barberis in N.L. laboratory and N.L. Adkins in C. Peterson’s laboratory for guidance on zebrafish experimental work and gel-filtration experiments, respectively. We thank G. Pazour for the gift of IFT88 antibody and GFP–IFT88 LLC-PK1, Flag–IFT52 IMCD stable cell lines, P. Wadsworth for the GFP– α -tubulin LLC-PK1 cell line and C. Desdouets, P. Denoulet and C. Janke for their gift of antibodies specific to IFT88 and polyglutamylated tubulin, respectively. Core resources supported by the Diabetes Endocrinology Research Center grant DK32520 were used; S.D. is a member of the UMass DERC (DK32520). This work was supported by financial support from the National Institutes of Health (GM51994) to S.D. and the Polycystic Kidney Disease Foundation to B.D.
Author information
Authors and Affiliations
Contributions
B.D. and S.D. wrote the manuscript. B.D. conceived and planned the experimental work. B.D. and A.B. carried out the experimental work and analysed the data. N.D.L. provided the zebrafish facility and helped plan and guide the zebrafish experimental work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1851 kb)
Supplementary Movie 1
Supplementary Information (MOV 509 kb)
Supplementary Movie 2
Supplementary Information (MOV 514 kb)
Supplementary Movie 3
Supplementary Information (MOV 728 kb)
Supplementary Movie 4
Supplementary Information (MOV 709 kb)
Supplementary Movie 5
Supplementary Information (MOV 40 kb)
Rights and permissions
About this article
Cite this article
Delaval, B., Bright, A., Lawson, N. et al. The cilia protein IFT88 is required for spindle orientation in mitosis. Nat Cell Biol 13, 461–468 (2011). https://doi.org/10.1038/ncb2202
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2202
This article is cited by
-
Depletion of Ift88 in thymic epithelial cells affects thymic synapse and T-cell differentiation in aged mice
Anatomical Science International (2022)
-
Genotoxic stress-activated DNA-PK-p53 cascade and autophagy cooperatively induce ciliogenesis to maintain the DNA damage response
Cell Death & Differentiation (2021)
-
The primary cilium dampens proliferative signaling and represses a G2/M transcriptional network in quiescent myoblasts
BMC Molecular and Cell Biology (2020)
-
Primary cilia-dependent signaling is involved in regulating mesenchymal stem cell proliferation and pluripotency maintenance
Journal of Molecular Histology (2020)
-
A reduction of primary cilia but not hedgehog signaling disrupts morphogenesis in testicular organoids
Cell and Tissue Research (2020)