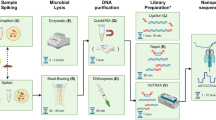
Abstract
Short-read, high-throughput sequencing technology cannot identify the chromosomal position of repetitive insertion sequences that typically flank horizontally acquired genes such as bacterial virulence genes and antibiotic resistance genes. The MinION nanopore sequencer can produce long sequencing reads on a device similar in size to a USB memory stick. Here we apply a MinION sequencer to resolve the structure and chromosomal insertion site of a composite antibiotic resistance island in Salmonella Typhi Haplotype 58. Nanopore sequencing data from a single 18-h run was used to create a scaffold for an assembly generated from short-read Illumina data. Our results demonstrate the potential of the MinION device in clinical laboratories to fully characterize the epidemic spread of bacterial pathogens.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Underwood, A.P. et al. Public health value of next-generation DNA sequencing of enterohemorrhagic Escherichia coli isolates from an outbreak. J. Clin. Microbiol. 51, 232–237 (2013).
Wain, J. & Mavrogiorgou, E. Next-generation sequencing in clinical microbiology. Expert Rev. Mol. Diagn. 13, 225–227 (2013).
Thomson, N. et al. The role of prophage-like elements in the diversity of Salmonella enterica serovars. J. Mol. Biol. 339, 279–300 (2004).
Livermore, D.M. & Wain, J. Revolutionising bacteriology to improve treatment outcomes and antibiotic stewardship. Infect Chemother. 45, 1–10 (2013).
Clarke, J. et al. Continuous base identification for single-molecule nanopore DNA sequencing. Nat. Nanotechnol. 4, 265–270 (2009).
Buckle, G.C., Walker, C.L. & Black, R.E. Typhoid fever and paratyphoid fever: Systematic review to estimate global morbidity and mortality for 2010. J. Glob. Health 2, 010401 (2012).
Wain, J., Hendriksen, R., Mikoleit, M., Keddy, K. & Ochiai, R. Typhoid fever. Lancet 10.1016/S0140-6736(13)62708-7 (21 October 2014).
Roumagnac, P. et al. Evolutionary history of Salmonella typhi. Science 314, 1301–1304 (2006).
Kariuki, S. et al. Typhoid in Kenya is associated with a dominant multidrug-resistant Salmonella enterica serovar Typhi haplotype that is also widespread in Southeast Asia. J. Clin. Microbiol. 48, 2171–2176 (2010).
Holt, K.E. et al. Temporal fluctuation of multidrug resistant salmonella typhi haplotypes in the mekong river delta region of Vietnam. PLoS Negl. Trop. Dis. 5, e929 (2011).
Holt, K.E. et al. High-resolution genotyping of the endemic Salmonella Typhi population during a Vi (typhoid) vaccination trial in Kolkata. PLoS Negl. Trop. Dis. 6, e1490 (2012).
Holt, K.E. et al. High-throughput sequencing provides insights into genome variation and evolution in Salmonella Typhi. Nat. Genet. 40, 987–993 (2008).
Holt, K.E. et al. Emergence of a globally dominant IncHI1 plasmid type associated with multiple drug resistant typhoid. PLoS Negl. Trop. Dis. 5, e1245 (2011).
Le, T.A. et al. Clonal expansion and microevolution of quinolone-resistant Salmonella enterica serotype typhi in Vietnam from 1996 to 2004. J. Clin. Microbiol. 45, 3485–3492 (2007).
Phan, M.D. et al. Variation in Salmonella enterica serovar typhi IncHI1 plasmids during the global spread of resistant typhoid fever. Antimicrob. Agents Chemother. 53, 716–727 (2009).
Frith, M.C., Hamada, M. & Horton, P. Parameters for accurate genome alignment. BMC Bioinformatics 11, 80 (2010).
Adey, A. et al. Rapid, low-input, low-bias construction of shotgun fragment libraries by high-density in vitro transposition. Genome Biol. 11, R119 (2010).
Bankevich, A. et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 19, 455–477 (2012).
Szczepanowski, R. et al. The 120 592 bp IncF plasmid pRSB107 isolated from a sewage-treatment plant encodes nine different antibiotic-resistance determinants, two iron-acquisition systems and other putative virulence-associated functions. Microbiology 151, 1095–1111 (2005).
Watson, M. et al. poRe: an R package for the visualization and analysis of nanopore sequencing data. Bioinformatics doi:10.1093/bioinformatics/btu590 (29 August 2014).
Loman, N.J. & Quinlan, A.R. Poretools: a toolkit for analyzing nanopore sequence data. Bioinformatics 30, 3399–3401 (2014).
Quick, J., Quinlan, A.R. & Loman, N.J. A reference bacterial genome dataset generated on the MinION portable single-molecule nanopore sequencer. GigaScience 3, 22 (2014).
Mikheyev, A.S. & Tin, M.M.Y. A first look at the Oxford Nanopore MinION sequencer. Mol. Ecol. Res. 14, 1097–1102 (2014).
Kim, K.E. et al. Long-read, whole genome shotgun sequence data for five model organisms. Preprint at http://biorxiv.org/content/early/2014/10/23/008037 (2014).
Chin, C.S. et al. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat. Methods 10, 563–569 (2013).
Parkhill, J. et al. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 413, 848–852 (2001).
Holt, K.E. et al. Pseudogene accumulation in the evolutionary histories of Salmonella enterica serovars Paratyphi A and Typhi. BMC Genomics 10, 36 (2009).
Grimont, A. & Weill, F. Antigenic Formulae of the Salmonella Serovars 9th edn. (World Health Organization, Geneva, 2007).
Callow, B. A new phage-typing scheme for Salmonella typhi-murium . J. Hyg. (Lond.) 57, 346–359 (1959).
Cock, P.J. et al. Biopython: freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics 25, 1422–1423 (2009).
Ewing, B. & Green, P. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 8, 175–185 (1998).
Acknowledgements
M. Day (Salmonella Reference Unit, Public Health England) for antibiotic susceptibility tests. J.O'G. and J.W. were funded by the University of East Anglia. We would like to thank Oxford Nanopore Technologies Ltd. for including us in the MinION Access Programme. We would also like to thank L. Nederbragt for a thorough review and contributions toward the presentation of Figure 1.
Author information
Authors and Affiliations
Contributions
P.M.A., S.N., T.D., J.W. and J.O'G. conceived the study, performed the analysis and wrote the first draft of the manuscript. J.O'G. and S.M. performed the MinION sequencing. P.M.A. and T.D. performed the bioinformatics analysis. S.N. performed the PCR analysis and coordinated the Illumina sequencing. P.M.A., T.D., S.R., W.R., J.W. and J.O'G. analyzed the resistance island structure and insertion site and devised the figures. All authors contributed to editing and data analysis of the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.O'G. is a participant of Oxford Nanopore's MinION Access Programme (MAP) and received the MinION device and flowcells used for this study free of charge.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5, Supplementary Tables 1–3 (PDF 345 kb)
Rights and permissions
About this article
Cite this article
Ashton, P., Nair, S., Dallman, T. et al. MinION nanopore sequencing identifies the position and structure of a bacterial antibiotic resistance island. Nat Biotechnol 33, 296–300 (2015). https://doi.org/10.1038/nbt.3103
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.3103
This article is cited by
-
Validation of Oxford nanopore sequencing for improved New World Leishmania species identification via analysis of 70-kDA heat shock protein
Parasites & Vectors (2023)
-
The diagnostic utility of nanopore targeted sequencing in suspected endophthalmitis
International Ophthalmology (2023)
-
Long-read metagenomic sequencing reveals shifts in associations of antibiotic resistance genes with mobile genetic elements from sewage to activated sludge
Microbiome (2022)
-
Antibiotic resistance in the environment
Nature Reviews Microbiology (2022)
-
Geometrical Model for the Growth Mechanism of Si Nanopores
Silicon (2022)