Abstract
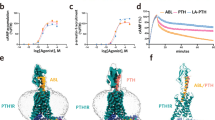
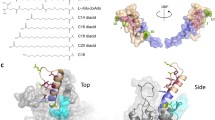
Systematic modification of the backbone of bioactive polypeptides through β-amino acid residue incorporation could provide a strategy for generating molecules with improved drug properties, but such alterations can result in lower receptor affinity and potency. Using an agonist of parathyroid hormone receptor-1 (PTHR1), a G protein–coupled receptor in the B-family, we present an approach for α→β residue replacement that enables both high activity and improved pharmacokinetic properties in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Accession codes
References
Vilardaga, J.P., Romero, G., Friedman, P.A. & Gardella, T.J. Cell. Mol. Life Sci. 68, 1–13 (2011).
Serada, M. et al. Xenobiotica 42, 398–407 (2012).
Uzawa, T., Hori, M., Ejiri, S. & Ozawa, H. Bone 16, 477–484 (1995).
Horne, W.S. et al. Proc. Natl. Acad. Sci. USA 106, 14751–14756 (2009).
Johnson, L.M. & Gellman, S.H. Methods Enzymol. 523, 407–429 (2013).
Wade, D. et al. Proc. Natl. Acad. Sci. USA 87, 4761–4765 (1990).
Chongsiriwatana, N.P. et al. Proc. Natl. Acad. Sci. USA 105, 2794–2799 (2008).
Porter, E.A., Wang, X.F., Lee, H.S., Weisblum, B. & Gellman, S.H. Nature 404, 565 (2000).
Schievano, E. et al. Biopolymers 70, 534–547 (2003).
Peggion, E. et al. Biochemistry 41, 8162–8175 (2002).
Underwood, C.R. et al. J. Biol. Chem. 285, 723–730 (2010).
Denton, E.V. et al. Org. Lett. 15, 5318–5321 (2013).
Boersma, M.D. et al. J. Am. Chem. Soc. 134, 315–323 (2012).
Pioszak, A.A. & Xu, H.E. Proc. Natl. Acad. Sci. USA 105, 5034–5039 (2008).
Hoare, S.R.J., Gardella, T.J. & Usdin, T.B. J. Biol. Chem. 276, 7741–7753 (2001).
Okazaki, M. et al. Proc. Natl. Acad. Sci. USA 105, 16525–16530 (2008).
Feinstein, T.N. et al. Nat. Chem. Biol. 7, 278–284 (2011).
Binkowski, B.F. et al. ACS Chem. Biol. 6, 1193–1197 (2011).
Winer, K.K., Sinaii, N., Peterson, D., Sainz, B. & Cutler, G.B. J. Clin. Endocrinol. Metab. 93, 3389–3395 (2008).
Kostenuik, P.J. et al. J. Bone Miner. Res. 22, 1534–1547 (2007).
Acknowledgements
This research was supported by the US National Institutes of Health (GM056414 (to S.H.G.) and NIDDK-11794 (to T.J.G.)). R.W.C. was supported in part by a National Institutes of Health Biotechnology Training grant (T32 GM008349).
Author information
Authors and Affiliations
Contributions
R.W.C. and S.H.G. developed the underlying molecular designs, all authors participated in the design and execution of experiments to assess new compounds, and all contributed to data interpretation. S.H.G. and R.W.C. wrote the paper with input from all other authors.
Corresponding authors
Ethics declarations
Competing interests
S.H.G. is a co-founder of Longevity Biotech, Inc., which is pursuing biomedical applications of α/β-peptides. S.H.G., R.W.C. and T.J.G. are co-inventors on a patent application covering the PTH analogs described here.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8, Supplementary Tables 1–4, and Supplementary Notes 1 and 2 (PDF 2214 kb)
Supplementary Data File 1
hPTHR1 cAMP Dose Response (XLSX 33 kb)
Supplementary Data File 2
PTH analog induced calcemic response (XLSX 49 kb)
Supplementary Data File 3
PTH analog pharmacokinetic profile data (XLSX 51 kb)
Source data
Rights and permissions
About this article
Cite this article
Cheloha, R., Maeda, A., Dean, T. et al. Backbone modification of a polypeptide drug alters duration of action in vivo. Nat Biotechnol 32, 653–655 (2014). https://doi.org/10.1038/nbt.2920
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.2920
This article is cited by
-
An amide to thioamide substitution improves the permeability and bioavailability of macrocyclic peptides
Nature Communications (2023)
-
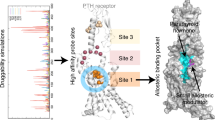
Precise druggability of the PTH type 1 receptor
Nature Chemical Biology (2022)
-
Improved GPCR ligands from nanobody tethering
Nature Communications (2020)
-
Peptidomimetics: A Synthetic Tool for Inhibiting Protein–Protein Interactions in Cancer
International Journal of Peptide Research and Therapeutics (2020)
-
Nonribosomal biosynthesis of backbone-modified peptides
Nature Chemistry (2018)