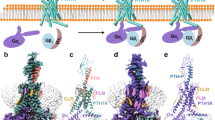
Abstract
Class B G protein-coupled receptors (GPCRs) are notoriously difficult to target by small molecules because their large orthosteric peptide-binding pocket embedded deep within the transmembrane domain limits the identification and development of nonpeptide small molecule ligands. Using the parathyroid hormone type 1 receptor (PTHR) as a prototypic class B GPCR target, and a combination of molecular dynamics simulations and elastic network model-based methods, we demonstrate that PTHR druggability can be effectively addressed. Here we found a key mechanical site that modulates the collective dynamics of the receptor and used this ensemble of PTHR conformers to identify selective small molecules with strong negative allosteric and biased properties for PTHR signaling in cell and PTH actions in vivo. This study provides a computational pipeline to detect precise druggable sites and identify allosteric modulators of PTHR signaling that could be extended to GPCRs to expedite discoveries of small molecules as novel therapeutic candidates.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Source of datasets analyzed during this study for figures (Figs. 1–6, Extended Data Figs. 2–4, and Supplementary Figs. 1–5) are provided with this paper. Materials and associated protocols will be made available to all qualified investigators from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Insel, P. A. et al. GPCRomics: an approach to discover GPCR drug targets. Trends Pharmacol. Sci. 40, 378–387 (2019).
Pandy-Szekeres, G. et al. GPCRdb in 2018: adding GPCR structure models and ligands. Nucleic Acids Res. 46, D440–D446 (2018).
Lyu, J. et al. Ultra-large library docking for discovering new chemotypes. Nature 566, 224–229 (2019).
Stein, R. M. et al. Virtual discovery of melatonin receptor ligands to modulate circadian rhythms. Nature 579, 609–614 (2020).
Basith, S. et al. Exploring G protein-coupled receptors (GPCRs) ligand space via cheminformatics approaches: impact on rational drug design. Front. Pharmacol. 9, 128 (2018).
Sutkeviciute, I. & Vilardaga, J. P. Structural insights into emergent signaling modes of G protein-coupled receptors. J. Biol. Chem. 295, 11626–11642 (2020).
Gardella, T. J. & Vilardaga, J. P. International Union of Basic and Clinical Pharmacology. XCIII. The parathyroid hormone receptors–family B G protein-coupled receptors. Pharmacol Rev 67, 310–337 (2015).
Cheloha, R. W., Gellman, S. H., Vilardaga, J. P. & Gardella, T. J. PTH receptor-1 signalling-mechanistic insights and therapeutic prospects. Nat. Rev. Endocrinol. 11, 712–724 (2015).
Kurkcuoglu, Z., Findik, D., Akten, E. D. & Doruker, P. How an inhibitor bound to subunit interface alters triosephosphate isomerase dynamics. Biophys. J. 109, 1169–1178 (2015).
Zhang, Y. et al. Intrinsic dynamics is evolutionarily optimized to enable allosteric behavior. Curr. Opin. Struct. Biol. 62, 14–21 (2019).
Kaynak, B. T., Bahar, I. & Doruker, P. Essential site scanning analysis: a new approach for detecting sites that modulate the dispersion of protein global motions. Comput. Struct. Biotechnol. J. 18, 1577–1586 (2020).
Zhao, L. H. et al. Structure and dynamics of the active human parathyroid hormone receptor-1. Science 364, 148–153 (2019).
Bahar, I., Jernigan, R. L. & Dill, K. A. Protein Actions: Principles & Modeling (Garland Science, Taylor & Francis Group, 2017).
Zhang, Y. et al. Intrinsic dynamics is evolutionarily optimized to enable allosteric behavior. Curr. Opin. Struct. Biol. 62, 14–21 (2020).
Le Guilloux, V., Schmidtke, P. & Tuffery, P. Fpocket: an open source platform for ligand pocket detection. BMC Bioinf. 10, 168 (2009).
Bakan, A., Nevins, N., Lakdawala, A. S. & Bahar, I. Druggability assessment of allosteric proteins by dynamics simulations in the presence of probe molecules. J. Chem. Theory Comput. 8, 2435–2447 (2012).
Lee, J. Y., Krieger, J. M., Li, H. & Bahar, I. Pharmmaker: pharmacophore modeling and hit identification based on druggability simulations. Protein Sci. https://doi.org/10.1002/pro.3732 (2019).
Sterling, T. & Irwin, J. J. ZINC 15–ligand discovery for everyone. J. Chem. Inf. Model. 55, 2324–2337 (2015).
Sunseri, J. & Koes, D. R. Pharmit: interactive exploration of chemical space. Nucleic Acids Res. 44, W442–W448 (2016).
Jean-Alphonse, F. G. et al. β2-adrenergic receptor control of endosomal PTH receptor signaling via Gβγ. Nat. Chem. Biol. 13, 259–261 (2017).
Hanyu, R. et al. Anabolic action of parathyroid hormone regulated by the beta2-adrenergic receptor. Proc. Natl Acad. Sci. USA 109, 7433–7438 (2012).
Feinstein, T. N. et al. Noncanonical control of vasopressin receptor type 2 signaling by retromer and arrestin. J. Bio. Chem. 288, 27849–27860 (2013).
Maeda, A. et al. Critical role of parathyroid hormone (PTH) receptor-1 phosphorylation in regulating acute responses to PTH. Proc. Natl Acad. Sci. USA 110, 5864–5869 (2013).
Okazaki, M. et al. Prolonged signaling at the parathyroid hormone receptor by peptide ligands targeted to a specific receptor conformation. Proc. Natl Acad. Sci. USA 105, 16525–16530 (2008).
Dean, T., Vilardaga, J. P., Potts, J. T. Jr. & Gardella, T. J. Altered selectivity of parathyroid hormone (PTH) and PTH-related protein (PTHrP) for distinct conformations of the PTH/PTHrP receptor. Mol. Endocrinol. 22, 156–166 (2008).
Ferrandon, S. et al. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat. Chem. Biol. 5, 734–742 (2009).
White, A. D. et al. Ca(2+) allostery in PTH-receptor signaling. Proc. Natl Acad. Sci. USA 116, 3294–3299 (2019).
White, A. D. et al. Gq/11-dependent regulation of endosomal cAMP generation by parathyroid hormone class B GPCR. Proc. Natl Acad. Sci. USA 117, 7455–7460 (2020).
Burnett-Bowie, S. M., Henao, M. P., Dere, M. E., Lee, H. & Leder, B. Z. Effects of hPTH(1-34) infusion on circulating serum phosphate, 1,25-dihydroxyvitamin D, and FGF23 levels in healthy men. J. Bone Miner. Res. 24, 1681–1685 (2009).
Cheng, Z. et al. Calcium-sensing receptors in chondrocytes and osteoblasts are required for callus maturation and fracture healing in mice. J. Bone Miner. Res. 35, 143–154 (2020).
Rickard, D. J. et al. Intermittent treatment with parathyroid hormone (PTH) as well as a non-peptide small molecule agonist of the PTH1 receptor inhibits adipocyte differentiation in human bone marrow stromal cells. Bone 39, 1361–1372 (2006).
Carter, P. H. et al. Discovery of a small molecule antagonist of the parathyroid hormone receptor by using an N-terminal parathyroid hormone peptide probe. Proc. Natl Acad. Sci. USA 104, 6846–6851 (2007).
Tamura, T. et al. Identification of an orally active small-molecule PTHR1 agonist for the treatment of hypoparathyroidism. Nat. Commun. 7, 13384 (2016).
Nishimura, Y. et al. Lead optimization and avoidance of reactive metabolite leading to PCO371, a potent, selective, and orally available human parathyroid hormone receptor 1 (hPTHR1) agonist. J. Med. Chem. 63, 5089–5099 (2020).
Hughes, J. P., Rees, S., Kalindjian, S. B. & Philpott, K. L. Principles of early drug discovery. Br. J. Pharmacol. 162, 1239–1249 (2011).
Kroeze, W. K. et al. PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome. Nat. Struct. Mol. Biol. 22, 362–369 (2015).
Bahar, I., Atilgan, A. R. & Erman, B. Direct evaluation of thermal fluctuations in proteins using a single-parameter harmonic potential. Fold Des. 2, 173–181 (1997).
Bakan, A., Meireles, L. M. & Bahar, I. ProDy: protein dynamics inferred from theory and experiments. Bioinformatics 27, 1575–1577 (2011).
Phillips, J. C. et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005).
Best, R. B. et al. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone phi, psi and side-chain chi(1) and chi(2) dihedral angles. J. Chem. Theory Comput. 8, 3257–3273 (2012).
Jorgensen, W. L., C. J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Vanommeslaeghe, K. & MacKerell, A. D. Jr. CHARMM additive and polarizable force fields for biophysics and computer-aided drug design. Biochim. Biophys. Acta 1850, 861–871 (2015).
Bakan, A. et al. Evol and ProDy for bridging protein sequence evolution and structural dynamics. Bioinformatics 30, 2681–2683 (2014).
Lee, J. et al. CHARMM-GUI input generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM simulations using the CHARMM36 additive force field. J. Chem. Theory Comput. 12, 405–413 (2016).
Huang, J. et al. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat. Methods 14, 71–73 (2017).
Gidon, A. et al. Endosomal GPCR signaling turned off by negative feedback actions of PKA and v-ATPase. Nat. Chem. Biol. 10, 707–709 (2014).
Feinstein, T. N. et al. Retromer terminates the generation of cAMP by internalized PTH receptors. Nat. Chem. Biol. 7, 278–284 (2011).
Cheloha, R. W., Maeda, A., Dean, T., Gardella, T. J. & Gellman, S. H. Backbone modification of a polypeptide drug alters duration of action in vivo. Nat. Biotechnol. 32, 653–655 (2014).
Cheng, Y. & Prusoff, W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 22, 3099–3108 (1973).
McElderry, J. D. et al. Tracking circadian rhythms of bone mineral deposition in murine calvarial organ cultures. J. Bone Mineral Res. 28, 1846–1854 (2013).
Acknowledgements
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Disease, the National Institute of General Medical Sciences, the National Institute on Drug Abuse and the National Center for Advancing Translational Sciences of the US National Institutes of Health (NIH) under grant awards nos. R01-DK116780 (to J.-P.V.), R01-DK122259 (to W.C. and J-P.V.), P41 GM103712 and R01 GM139297 (to I.B. and P.D.), P01-DK011794 (to T.J.G.) and by the University of Pittsburgh Clinical and Translational Science Institute (grant no. NIH UL1TR001857).
Author information
Authors and Affiliations
Contributions
A.D.W., S.L., S.Savransky, S.Sanker and K.A.P. performed signaling studies. A.D.W., I.S. and J.-P.V. performed signaling data analyses. J.Y.L. and H.L. performed druggability simulations. J.Y.L. performed pharmacophore modeling. B.K. and P.D. performed ESSA. T.J.G. provided guidance for radioligand binding assays. L.J.C. conducted docking studies. W.C., C.S.M. and C.T. conducted mouse studies. I.S., I.B. and J.-P.V. oversaw the project, designed the study and provided guidance. J.-P.V., I.B., I.S. and A.D.W. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
I.S., J.Y.L., B.K., I.B. and J.-P.V. acknowledge potential competing financial interests. No conflicts of interest were disclosed by other authors.
Additional information
Peer review information Nature Chemical Biology thanks Lei Shi, Celine Valant and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Close-up view of the pocket at the EC vestibule.
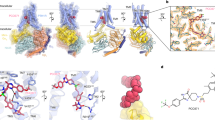
The pocket is lined by TM1 (red) and TM2 (cyan). Essential residues R1811.33b and Y2452.72b, whose interaction with ligands is predicted to affect the global dynamics of the overall PTHR, are shown in yellow sticks. Two additional residues, D252ECL1 and F1841.36b are also shown, which may help attract or coordinate the ligands through electrostatic interactions. The role of F184 in complementing R1811.33b and Y2452.72b to line a druggable pocket is further corroborated by druggability simulations.
Extended Data Fig. 2 Probe molecules used in druggability simulations.
(a) Seven probe molecules used in PTHR druggability simulations. The structure of each probe is shown along with its 4-letter acronym, its name and main chemical properties. Also shown is the percentage of drugs containing those fragments based on the SMILES data of 2,453 approved drugs in DrugBank. (b) An example of a common drug, benzyl-penicillin, containing four types of fragments represented by the probe molecules. (c) Binding score profile for PTHR residues near Site 1 (labeled along the abscissa), evaluated for different probe molecules (bars in different colors). The binding score is defined as Σ (1/𝑑ki)2 where k is frame/snapshot index and 𝑑ki is corresponding distance between the probe and the residue i summed over all snapshots n (12,000 compiled from six independent runs), provided that they make atom-atom contacts of 𝑑ki < 4.0 Å. Residues with score above 1500/Å2 (dashed line) are selected as high affinity residues for each probe type: R1811.33b for acetate, and F1841.36b and Y2452.72b for isopropanol. (d) Close-up view of site 1 preferentially sampled by three probes (two isopropanol and an acetic acid, shown in yellow and red sticks respectively), and associated residues R1811.33b, F1841.36b and Y2452.72b (right). (e, f) Construction of pharmacophore model (PM) composed of a hydrogen bond acceptor, a negatively charged region, and two hydrophobic sites (spheres), based on the preferential positions observed in (c), and overlay of a hit compound Pitt12 (aquamarine sticks, extracted from the ZINC database) and the PM. The box on the left in panel (f) shows the partial charges for the carboxylate group atoms O, C, O’, and H in Pitt12 in its protonated and deprotonated forms. In line with the use of acetate probe molecule to construct the PM, we used the deprotonated form in further analyses and simulations.
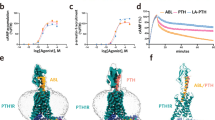
Extended Data Fig. 3 Actions of Pitt8 on PTH signaling.
(a, b) Binding isotherms showing competitive inhibition of radio-labeled peptide ligand binding to PTHR by PTH or Pitt8, using plasma membrane extracts from HEK293 cells expressing recombinant PTHR. Inhibition of [125I]-LAPTH binding to G protein-independent conformational R0 state of PTHR (that is, in the absence of G proteins) by PTH1-34 (black) or Pitt8 (pink) (a). Inhibition of [125I]-M-PTH1-15 binding to G protein-dependent RG conformational state of PTHR (that is, in the presence of G proteins) by M-PTH1-15 (black) or Pitt8 (pink). Mean ± s.d. of N = 6 experiments. (c) Concentration-response curves for cAMP production by PTH alone or together with Pitt8. Data are mean ± s.e.m. of N = 3 independent experiments. (d, e) Averaged cAMP time-courses following brief stimulation with 1 nM PTH without (Ctrl, black) or with 10 µM Pitt12 measured by FRET changes from HEK293 cells stably expressing PTHR and a FRET-based cAMP sensor EpacCFP/YFP in the absence (d) or presence of the dominant-negative dynamin mutant (DynK44A) tagged with RFP (e). (f) Time course of β-arrestin 2 interaction with PTHR measured by FRET in HEK293 cells transiently expressing PTHRCFP and βarr-2YFP following brief stimulation with 10 nM PTH without (Ctrl) or with 10 µM Pitt8. Cells were continuously perfused with control buffer or PTH alone or together with Pitt8 (horizontal bar). Data are the mean ± s.e.m. of N = 3 experiments with n = 52 (DMSO) and 47 (Pitt8) cells examined. (g) Time courses of Ca2+ release in response to PTH (100 nM) with or without Pitt8 (10 µM) in live HEK-293 cells expressing recombinant PTHR. Data are the mean ± s.e.m. of N = 3 experiments with n = 32 (DMSO) and 32 (Pitt8) cells examined.
Extended Data Fig. 4 Statistics for integrated responses from Fig. 3 and Extended Data Fig. 3.
(a) pKi values for Pitt12 for the RG state were: -10.77 ± 0.15 (M-PTH1-15) and -4.39 ± 0.96 (Pitt12); for the R0 state: -8.26 ± 0.14 (PTH1-34) and -5.15 ± 0.63. pKi values for Pitt8 the RG state were: -10.77 ± 0.15 (M-PTH1-15) and -5.25 ± 0.48 (Pitt8); for the R0 state: -8.26 ± 0.14 (PTH1-34) and -4.79 ± 0.30 (Pitt8). Mean ± s.d. of N = 6 experiments. (b) Quantitation of cAMP responses by measuring the area under the curve (A.U.C.) from 0 to 20 min for Fig. 3e,f, and Extended Data Fig. 3d,e. Data are mean ± s.d. of N = 6 (PTH), 3 (Pitt12), and 3 (Pitt8) experiments for control, and N = 4 (PTH), 4 (Pitt12), and 4 (Pitt8) experiments for DynK44A. NS, not significant, **P < 0.002 and ***P < 0.0002 by two-way ANOVA with Tukey-Kramer post-hoc test. (c, d) Data are from Fig. 3h and Extended Data Fig. 3g. Mean ± s.e.m. of N = 3 independent experiments. P values were assessed by two-tailed Student’s t-test with *P < 0.05, **P < 0.005.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2 and Figs. 1–5.
Supplementary Video 1
A video from initial druggability simulations showing persistent contacts between Pitt12 and PTHR residues E1801.32b and R1811.33b.
Supplementary Data 1
Source data spreadsheet for Supplementary Fig. 1.
Supplementary Data 2
Source data spreadsheet for Supplementary Fig. 2.
Supplementary Data 3
Source data spreadsheet for Supplementary Fig. 3.
Supplementary Data 4
Source data spreadsheet for Supplementary Fig. 4.
Supplementary Data 5
Source data spreadsheet for Supplementary Fig. 5.
Source data
Source Data Fig. 1
Source data.
Source Data Fig. 2
Source data.
Source Data Fig. 3
Source data.
Source Data Fig. 4
Source data.
Source Data Fig. 5
Source data.
Source Data Fig. 6
Source data.
Source Data Extended Data Fig. 2
Source data.
Source Data Extended Data Fig. 3
Source data.
Source Data Extended Data Fig. 4
Source data.
Rights and permissions
About this article
Cite this article
Sutkeviciute, I., Lee, J.Y., White, A.D. et al. Precise druggability of the PTH type 1 receptor. Nat Chem Biol 18, 272–280 (2022). https://doi.org/10.1038/s41589-021-00929-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-021-00929-w
This article is cited by
-
G protein-coupled receptors (GPCRs): advances in structures, mechanisms, and drug discovery
Signal Transduction and Targeted Therapy (2024)