Abstract
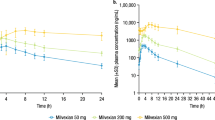
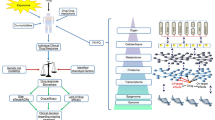
In 2010, the US Food and Drug Administration (FDA) approved a generic low-molecular-weight heparin without clinical safety or efficacy data under the Abbreviated New Drug Application (ANDA) pathway. To enable a determination of active ingredient sameness of generic and innovator enoxaparin products, the FDA developed a scientifically rigorous approach based on five criteria: first, equivalence of physicochemical properties; second, equivalence of heparin source material and mode of depolymerization; third, equivalence in disaccharide building blocks, fragment mapping and sequence of oligosaccharide species; fourth, equivalence in biological and biochemical assays; and finally, equivalence of in vivo pharmacodynamic profile. In addition to fulfillment of these criteria, FDA also used in vitro, ex vivo and model animal data to ensure there was no increased immunogenicity risk of the generic enoxaparin product relative to the brand name product. The approval of the highly complex enoxaparin product using this framework under the ANDA pathway represents a major development. It also suggests that analytical and scientific advancements may in certain cases allow the elimination of unnecessary in vivo testing in animals and humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
US Food and Drug Administration. FDA approves first generic enoxaparin sodium injection. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm220092.htm (Accessed 24 January 2012).
Hirsh, J. et al. Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest 119, 64S–94S (2001).
21 CFR 320.1(c). (Code of Federal Regulations; Title 21, Food and drugs; Part 320, Bioavailability and bioequivalence requirements; FDA HHS, 2012).
21 CFR 320.1(e). (Code of Federal Regulations; Title 21, Food and drugs; Part 320, Bioavailability and bioequivalence requirements; FDA HHS, 2012).
21 CFR 320.22(b)(1). (Code of Federal Regulations; Title 21, Food and drugs; Part 320, Bioavailability and bioequivalence requirements; FDA HHS, 2012).
Kozlowski, S., Woodcock, J., Midthun, K. & Sherman, R.B. Developing the nation's biosimilars program. N. Engl. J. Med. 365, 385–388 (2011).
Linhardt, R.J. & Gunay, N.S. Production and chemical processing of low molecular weight heparins. Semin. Thromb. Hemost. 25 (suppl. 3), 5–16 (1999).
Sasisekharan, R. & Venkataraman, G. Heparin and heparan sulfate: biosynthesis, structure and function. Curr. Opin. Chem. Biol. 4, 626–631 (2000).
Ernst, S., Langer, R., Cooney, C.L. & Sasisekharan, R. Enzymatic degradation of glycosaminoglycans. Crit. Rev. Biochem. Mol. Biol. 30, 387–444 (1995).
Guerrini, M., Bisio, A. & Torri, G. Combined quantitative H-1 and C-13 nuclear magnetic resonance spectroscopy for characterization of heparin preparations. Semin. Thromb. Hemost. 27, 473–482 (2001).
Nugent, M.A. Heparin sequencing brings structure to the function of complex oligosaccharides. Proc. Natl. Acad. Sci. USA 97, 10301–10303 (2000).
Linhardt, R.J. 2003 Claude S. Hudson Award address in carbohydrate chemistry. Heparin: structure and activity. J. Med. Chem. 46, 2551–2564 (2003).
Capila, I. & Linhardt, R.J. Heparin-protein interactions. Angew. Chem. Int. Ed. Engl. 41, 390–412 (2002).
Pharmacopeia, US 35–NF30. Official monographs: heparin sodium. http://www.uspnf.com/uspnf (Accessed December 15, 2012).
US Food and Drug Administration. Product Labeling for Lovenox (enoxaparin sodium injection). http://www.accessdata.fda.gov/scripts/cder/drugsatfda/ (Accessed January 25, 2012).
Hirsh, J. et al. Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest 119, 64S–94S (2001).
Debrie, R. Mixtures of particular LMW heparinic polysaccharides for the prophylaxis/treatment of acute thrombotic events. US patent no. 5,389,618 (1995).
Torri, G. & Guerrini, M. in NMR Spectroscopy in Pharmaceutical Analysis (eds. Holzgrabe, U., Wawer, I. & Diehl, B.) 407–427 (Elsevier, Oxford; 2008).
Merli, G.J., Vanscoy, G.J., Rihn, T.L., Groce, J.B. III & McCormick, W. Applying scientific criteria to therapeutic interchange: a balanced analysis of low-molecular-weight heparins. J. Thromb. Thrombolysis 11, 247–259 (2001).
US Food and Drug Administration. Generic enoxaparin questions and answers (updated July 23, 2010). http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm220037.htm (Accessed December 15, 2012).
Nightingale, S.L. Appropriate use of low-molecular-weight heparins (LMWHs). JAMA 270, 1672 (1993).
Planes, A. et al. Prevention of deep vein thrombosis after hip replacement–comparison between two low-molecular heparins, tinzaparin and enoxaparin. Thromb. Haemost. 81, 22–25 (1999).
US Food and Drug Administration. Response to Citizen Petition, Docket No. FDA-2003-P-0273. http://www.regulations.gov (2010).
Thanawiroon, C., Rice, K.G., Toida, T. & Linhardt, R.J. Liquid chromatography/mass spectrometry sequencing approach for highly sulfated heparin-derived oligosaccharides. J. Biol. Chem. 279, 2608–2615 (2004).
Mourier, P.A.J. & Viskov, C. Chromatographic analysis and sequencing approach of heparin oligosaccharides using cetyltrimethylammonium dynamically coated stationary phases. Anal. Biochem. 332, 299–313 (2004).
Chuang, W.L., Christ, M.D. & Rabenstein, D.L. Determination of the primary structures of heparin- and heparan sulfate-derived oligosaccharides using band-selective homonuclear-decoupled two dimensional H-1 NMR experiments. Anal. Chem. 73, 2310–2316 (2001).
Sundaram, M. et al. Rational design of low-molecular weight heparins with improved in vivo activity. Proc. Natl. Acad. Sci. USA 100, 651–656 (2003).
Imanari, T., Toyoda, H., Yamamoto, H., Ogino, N. & Toida, T. Rapid and sensitive analysis of disaccharide composition in heparin and heparan sulfate by reversed-phase ion-pair chromatography on a 2 mm porous silica gel column. J. Chromatogr. A 830, 197–201 (1999).
Mourier, P. & Viskov, C. Method for determining specific groups constituting heparins or low molecular weight heparins. US patent no. US2005/0119477 A1 (2005).
Myette, J.R. et al. Molecular cloning of the heparin/heparan sulfate Delta 4,5 unsaturated glycuronidase from Flavobacterium heparinum, its recombinant expression in Escherichia coli, and biochemical determination of its unique substrate specificity. Biochemistry 41, 7424–7434 (2002).
Stringer, S.E., Kandola, B.S., Pye, D.A. & Gallagher, J.T. Heparin sequencing. Glycobiology 13, 97–107 (2003).
Linhardt, R.J. et al. Mapping and quantification of the major oligosaccharide components of heparin. Biochem. J. 254, 781–787 (1988).
Chuang, W.L., McAllister, H. & Rabenstein, D.L. Chromatographic methods for product-profile analysis and isolation of oligosaccharides produced by heparinase-catalyzed depolymerization of heparin. J. Chromatogr. A 932, 65–74 (2001).
Shriver, Z. et al. Sequencing of 3-O sulfate containing heparin decasaccharides with a partial antithrombin III binding site. Proc. Natl. Acad. Sci. USA 97, 10359–10364 (2000).
Shriver, Z. et al. Sequencing of 3-O sulfate containing heparin decasaccharides with a partial antithrombin III binding site. Proc. Natl. Acad. Sci. USA 97, 10359–10364 (2000).
Venkataraman, G., Shriver, Z., Raman, R. & Sasisekharan, R. Sequencing complex polysaccharides. Science 286, 537–542 (1999).
Turnbull, J.E., Hopwood, J.J. & Gallagher, J.T. A strategy for rapid sequencing of heparan sulfate and heparin saccharides. Proc. Natl. Acad. Sci. USA 96, 2698–2703 (1999).
Yamada, S. et al. Structural studies of octasaccharides derived from the low-sulfated repeating disaccharide region and octasaccharide serines derived from the protein linkage region of porcine intestinal heparin. Biochemistry 38, 838–847 (1999).
Pharmacopeia, US 35–NF30. Official monographs: enoxaparin sodium. http://www.uspnf.com/uspnf (Accessed December 15, 2012).
Hirsh, J. & Raschke, R. Heparin and low-molecular-weight heparin–the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126, 188s–203s (2004).
Chuang, Y.J., Swanson, R., Raja, S.M. & Olson, S.T. Heparin enhances the specificity of antithrombin for thrombin and factor Xa independent of the reactive center loop sequence. Evidence for an exosite determinant of factor Xa specificity in heparin-activated antithrombin. J. Biol. Chem. 276, 14961–14971 (2001).
Bisio, A. et al. Structural features of low-molecular-weight heparins affecting their affinity to antithrombin. Thromb. Haemost. 102, 865–873 (2009).
Guerrini, M. et al. Effects on molecular conformation and anticoagulant activities of 1,6-anhydrosugars at the reducing terminal of antithrombin-binding octasaccharides isolated from low-molecular-weight heparin enoxaparin. J. Med. Chem. 53, 8030–8040 (2010).
Rosenberg, R.D. Actions and interactions of antithrombin and heparin. N. Engl. J. Med. 292, 146–151 (1975).
Jeske, W. & Fareed, J. In vitro studies on the biochemistry and pharmacology of low molecular weight heparins. Semin. Thromb. Hemost. 25 (suppl. 3), 27–33 (1999).
Eriksson, B.I. et al. A comparative study of three low-molecular weight heparins (LMWH) and unfractionated heparin (UH) in healthy volunteers. Thromb. Haemost. 73, 398–401 (1995).
Samama, M.M. & Gerotziafas, G.T. Comparative pharmacokinetics of LMWHs. Semin. Thromb. Hemost. 26 (suppl. 1), 31–38 (2000).
Martel, N., Lee, J. & Wells, P.S. Risk for heparin-induced thrombocytopenia. with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood 106, 2710–2715 (2005).
Arepally, G.M. & Ortel, T.L. Heparin-induced thrombocytopenia. Annu. Rev. Med. 61, 77–90 (2010).
Greinacher, A. et al. Heparin-induced thrombocytopenia: a prospective study on the incidence, platelet-activating capacity and clinical significance of antiplatelet factor 4/heparin antibodies of the IgG, IgM, and IgA classes. J. Thromb. Haemost. 5, 1666–1673 (2007).
Girolami, B. & Girolami, A. Heparin-induced thrombocytopenia: a review. Semin. Thromb. Hemost. 32, 803–809 (2006).
Selleng, S. et al. Incidence and clinical relevance of anti-platelet factor 4/heparin antibodies before cardiac surgery. Am. Heart J. 160, 362–369 (2010).
Hursting, M.J. et al. Platelet factor 4/heparin antibodies in blood bank donors. Am. J. Clin. Pathol. 134, 774–780 (2010).
Newman, P.M., Swanson, R.L. & Chong, B.H. Heparin-induced thrombocytopenia: IgG binding to PF4-heparin complexes in the fluid phase and cross-reactivity with low molecular weight heparin and heparinoid. Thromb. Haemost. 80, 292–297 (1998).
Greinacher, A., Alban, S., Dummel, V., Franz, G. & Muellereckhardt, C. Characterization of the structural requirements for a carbohydrate-based anticoagulant with a reduced risk of inducing the immunological type of heparin-associated thrombocytopenia. Thromb. Haemost. 74, 886–892 (1995).
Maccarana, M. & Lindahl, U. Mode of interaction between platelet factor-Iv and heparin. Glycobiology 3, 271–277 (1993).
Suvarna, S. et al. Determinants of PF4/heparin immunogenicity. Blood 110, 4253–4260 (2007).
Pattnaik, P. Surface plasmon resonance—applications in understanding receptor-ligand interaction. Appl. Biochem. Biotechnol. 126, 79–92 (2005).
Kamberi, M. et al. Analysis of non-covalent aggregation of synthetic hPTH (1–34) by size-exclusion chromatography and the importance of suppression of non-specific interactions for a precise quantitation. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 810, 151–155 (2004).
Lebowitz, J., Lewis, M.S. & Schuck, P. Modern analytical ultracentrifugation in protein science: a tutorial review. Protein Sci. 11, 2067–2079 (2002).
Levin, S. Field flow fractionation in biomedical analysis. Biomed. Chromatogr. 5, 133–138 (1991).
Greinacher, A. et al. Close approximation of two platelet factor 4 tetramers by charge neutralization forms the antigens recognized by HIT antibodies. Arterioscler. Thromb. Vasc. Biol. 26, 2386–2393 (2006).
Rauova, L. et al. Ultralarge complexes of PF4 and heparin are central to the pathogenesis of heparin-induced thrombocytopenia. Blood 105, 131–138 (2005).
Heinzelmann, M. et al. Heparin and enoxaparin enhance endotoxin-induced tumor necrosis factor-alpha production in human monocytes. Ann. Surg. 229, 542–550 (1999).
Hochart, H. et al. Concentration-dependent roles for heparin in modifying lipopolysaccharide-induced activation of mononuclear cells in whole blood. Thromb. Haemost. 99, 570–575 (2008).
Maddineni, J. et al. Product individuality of commercially available low-molecular-weight heparins and their generic versions: therapeutic implications. Clin. Appl. Thromb. Hemost. 12, 267–276 (2006).
Ofosu, F.A. A review of the two major regulatory pathways for non-proprietary low-molecular-weight heparins. Thromb. Haemost. 107, 201–214 (2012).
European Medicines Agency. Guideline on non-clinical and clinical development of similar biological medicinal products containing low-molecular-weight heparins, 2013. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/01/WC500138309.pdf (Accessed February 7, 2013).
Harenberg, J. et al. Recommendations on biosimilar low-molecular-weight heparins. J. Thromb. Haemost. 7, 1222–1225 (2009).
Kalodiki, E. & Leong, W. SASAT (South Asian Society on Atherosclerosis & Thrombosis) proposal for regulatory guidelines for generic low-molecular weight heparins (LMWHs). Clin. Appl. Thromb. Hemost. 15, 8–11 (2009).
Harenberg, J. Differences of present recommendations and guidelines for generic low-molecular-weight heparins: is there room for harmonization. Clin. Appl. Thromb. Hemost. 17, E158–E164 (2011).
Harenberg, J. Overview on guidelines and recommendations for generic low-molecular-weight heparins. Thromb. Res. 127 (suppl. 3), S100–S104 (2011).
Maccarana, M., Casu, B. & Lindahl, U. Minimal sequence in heparin heparan-sulfate required for binding of basic fibroblast growth-factor. J. Biol. Chem. 269, 3903 (1994).
Cohen, M., Jeske, W.P., Nicolau, J.C., Montalescot, G. & Fareed, J. US Food and Drug Administration approval of generic versions of complex biologics: implications for the practicing physician using low molecular weight heparins. J. Thromb. Thrombolysis 33, 230–238 (2012).
Garcia-Arieta, A. & Blazquez, A. Regulatory considerations for generic or biosimilar low molecular weight heparins. Curr. Drug Discov. Technol. 9, 137–142 (2012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Lee, S., Raw, A., Yu, L. et al. Scientific considerations in the review and approval of generic enoxaparin in the United States. Nat Biotechnol 31, 220–226 (2013). https://doi.org/10.1038/nbt.2528
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.2528
This article is cited by
-
Commercial Low Molecular Weight Heparins — Patent Ecosystem and Technology Paradigm for Quality Characterization
Journal of Pharmaceutical Innovation (2023)
-
Platelet factor 4 polyanion immune complexes: heparin induced thrombocytopenia and vaccine-induced immune thrombotic thrombocytopenia
Thrombosis Journal (2021)
-
Therapeutic strategies for thrombosis: new targets and approaches
Nature Reviews Drug Discovery (2020)
-
Salt-free fractionation of complex isomeric mixtures of glycosaminoglycan oligosaccharides compatible with ESI-MS and microarray analysis
Scientific Reports (2019)