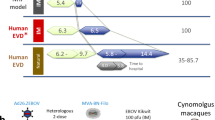
Abstract
Without an approved vaccine or treatments, Ebola outbreak management has been limited to palliative care and barrier methods to prevent transmission. These approaches, however, have yet to end the 2014 outbreak of Ebola after its prolonged presence in West Africa. Here we show that a combination of monoclonal antibodies (ZMapp), optimized from two previous antibody cocktails, is able to rescue 100% of rhesus macaques when treatment is initiated up to 5 days post-challenge. High fever, viraemia and abnormalities in blood count and blood chemistry were evident in many animals before ZMapp intervention. Advanced disease, as indicated by elevated liver enzymes, mucosal haemorrhages and generalized petechia could be reversed, leading to full recovery. ELISA and neutralizing antibody assays indicate that ZMapp is cross-reactive with the Guinean variant of Ebola. ZMapp exceeds the efficacy of any other therapeutics described so far, and results warrant further development of this cocktail for clinical use.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bausch, D. G., Sprecher, A. G., Jeffs, B. & Boumandouki, P. Treatment of Marburg and Ebola hemorrhagic fevers: a strategy for testing new drugs and vaccines under outbreak conditions. Antiviral Res. 78, 150–161 (2008)
Baize, S. et al. Emergence of Zaire Ebola virus disease in Guinea — preliminary report. N. Engl. J. Med. http://dx.doi.org/10.1056/NEJMoa1404505 (2014)
WHO. Ebola virus disease (EVD) http://www.who.int/csr/don/archive/disease/ebola/en/ (accessed, 15 August 2014)
CDC. Chronology of Ebola Hemorrhagic Fever Outbreaks, http://www.cdc.gov/vhf/ebola/resources/outbreak-table.html (accessed, 15 August 2014)
Reliefweb. W. African Ebola epidemic ‘likely to last months': UN http://reliefweb.int/report/guinea/w-african-ebola-epidemic-likely-last-months-un (7 March 2014)
Clark, D. V., Jahrling, P. B. & Lawler, J. V. Clinical management of filovirus-infected patients. Viruses 4, 1668–1686 (2012)
Guimard, Y. et al. Organization of patient care during the Ebola hemorrhagic fever epidemic in Kikwit, Democratic Republic of the Congo, 1995. J. Infect. Dis. 179 (Suppl. 1). S268–S273 (1999)
Hensley, L. E. et al. Recombinant human activated protein C for the postexposure treatment of Ebola hemorrhagic fever. J. Infect. Dis. 196 (Suppl 2). S390–S399 (2007)
Geisbert, T. W. et al. Treatment of Ebola virus infection with a recombinant inhibitor of factor VIIa/tissue factor: a study in rhesus monkeys. Lancet 362, 1953–1958 (2003)
Geisbert, T. W. et al. Postexposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: a proof-of-concept study. Lancet 375, 1896–1905 (2010)
Warren, T. K. et al. Advanced antisense therapies for postexposure protection against lethal filovirus infections. Nature Med. 16, 991–994 (2010)
Feldmann, H. et al. Effective post-exposure treatment of Ebola infection. PLoS Pathog. 3, e2 (2007)
Olinger, G. G., Jr et al. Delayed treatment of Ebola virus infection with plant-derived monoclonal antibodies provides protection in rhesus macaques. Proc. Natl Acad. Sci. USA 109, 18030–18035 (2012)
Qiu, X. et al. Successful treatment of ebola virus-infected cynomolgus macaques with monoclonal antibodies. Sci. Transl. Med. 4, 138ra181 (2012)
Pettitt, J. et al. Therapeutic intervention of Ebola virus infection in rhesus macaques with the MB-003 monoclonal antibody cocktail. Sci. Transl. Med. 5, 199ra113 (2013)
Qiu, X. et al. mAbs and Ad-vectored IFN-α therapy rescue Ebola-infected nonhuman primates when administered after the detection of viremia and symptoms. Sci. Transl. Med. 5, 207ra143 (2013)
Giritch, A. et al. Rapid high-yield expression of full-size IgG antibodies in plants coinfected with noncompeting viral vectors. Proc. Natl Acad. Sci. USA 103, 14701–14706 (2006)
Jahrling, P. B. et al. Evaluation of immune globulin and recombinant interferon-alpha2b for treatment of experimental Ebola virus infections. J. Infect. Dis. 179 (Suppl 1). S224–S234 (1999)
Qiu, X. et al. Ebola GP-specific monoclonal antibodies protect mice and guinea pigs from lethal Ebola virus infection. PLoS Negl. Trop. Dis. 6, e1575 (2012)
Wilson, J. A. et al. Epitopes involved in antibody-mediated protection from Ebola virus. Science 287, 1664–1666 (2000)
Qiu, X. et al. Characterization of Zaire ebolavirus glycoprotein-specific monoclonal antibodies. Clin. Immunol. 141, 218–227 (2011)
Dye, J. M. et al. Postexposure antibody prophylaxis protects nonhuman primates from filovirus disease. Proc. Natl Acad. Sci. USA 109, 5034–5039 (2012)
Wong, G. et al. Immune parameters correlate with protection against ebola virus infection in rodents and nonhuman primates. Sci. Transl. Med. 4, 158ra146 (2012)
Marzi, A. et al. Antibodies are necessary for rVSV/ZEBOV-GP-mediated protection against lethal Ebola virus challenge in nonhuman primates. Proc. Natl Acad. Sci. USA 110, 1893–1898 (2013)
ProMEDmail.org. Ebola virus disease - West Africa (117): WHO, Nigeria, Liberia, drug, more. http://www.promedmail.org/direct.php?id=2666073 (6 August 2014)
NC3RS. Practical blood sample volumes for laboratory animals, domestic species and non-human primates. http://www.nc3rs.org.uk/bloodsamplingmicrosite/page.asp?id=426 (accessed, 3 August 2014)
Qiu, X. et al. Sustained protection against Ebola virus infection following treatment of infected nonhuman primates with ZMAb. Sci. Rep. 3, 3365 (2013)
Wong, G. et al. Immunization with vesicular stomatitis virus vaccine expressing the Ebola glycoprotein provides sustained long-term protection in rodents. Vaccine http://dx.doi.org/10.1016/j.vaccine.2014.08.028 (in the press)
Mupapa, K. et al. Treatment of Ebola hemorrhagic fever with blood transfusions from convalescent patients. International Scientific and Technical Committee. J. Infect. Dis. 179 (Suppl. 1). S18–S23 (1999)
UPMChealthsecurity.org. Next-Generation Monoclonal Antibodies: Challenges and Opportunitieshttp://www.upmchealthsecurity.org/our-work/pubs_archive/pubs-pdfs/2013/2013-02-04-next-gen-monoclonal-antibodies.pdf (UPMC Center for Biosecurity, 2013)
Zeitlin, L. et al. Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant. Proc. Natl Acad. Sci. USA 108, 20690–20694 (2011)
Connolly, B. M. et al. Pathogenesis of experimental Ebola virus infection in guinea pigs. J. Infect. Dis. 179 (Suppl. 1). S203–S217 (1999)
Reed, L. J. & Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27, 493–497 (1938)
Acknowledgements
The authors thank K. Tierney, A. Grolla, S. Jones, J. Dong and D. Kobasa for their technical assistance, V. Klimyuk and Y. Gleba for access to the magnICON expression system, and H. Steinkellner for access to transgenic N. benthamiana. This work was supported by the Defense Threat Reduction Agency (DTRA contract HDTRA1-13-C-0018), the National Institutes of Health (U19AI109762), the Public Health Agency of Canada (PHAC), and a Canadian Safety and Security Program (CSSP) grant to G.P.K. and X.Q. G.W. is the recipient of a Doctoral Research Award from the Canadian Institute for Health Research (CIHR).
Author information
Authors and Affiliations
Contributions
X.Q., G.P.K. and L.Z. designed the experiments. X.Q., G.W., J.A., A.B., L.F., J.B.A., H.F., H.W., J.A., J. P., G.G.O. and G.P.K. performed the experiments. X.Q., G.W., J.A., K.W., B.X., J.E.S., L.Z. and G.P.K. wrote the manuscript. E.H., A.J., J.M., K.S., O.B., N.B., C.G., D.K., M.H.P., J.V., K.W. and L.Z. contributed reagents for this study.
Corresponding authors
Ethics declarations
Competing interests
Her Majesty the Queen in right of Canada holds a patent on mAbs 2G4, and 4G7, PCT/CA2009/000070, “Monoclonal antibodies for Ebola and Marburg viruses.” K.W. and L.Z. are the owners of Mapp Biopharmaceutical Inc. The authors declare no other competing interests.
Extended data figures and tables
Extended Data Figure 1 Clinical scores for each ZMapp-treated group.
Arrows indicate treatment days. Dashed line represents humane endpoint threshold. Faded symbols/lines are the other two treatment groups, for comparison. Control group (Group G) is shown in black on all three panels. a, Clinical score of Group D (blue); b, clinical score of Group E (orange); c, clinical score of Group F (green).
Extended Data Figure 2 Viraemia for each ZMapp-treated group.
Arrows indicate treatment days. Faded symbols/lines are the other two treatment groups, for comparison. Control group (Group G) is shown in black on all three panels. a, TCID50 of Group D (blue); b, TCID50 of Group E (orange); c, TCID50 of Group F (green). d, Viraemia by RT–qPCR of Group D (blue); e, viraemia by RT–qPCR of Group E (orange); f, viraemia by RT–qPCR of Group F (green).
Rights and permissions
About this article
Cite this article
Qiu, X., Wong, G., Audet, J. et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 514, 47–53 (2014). https://doi.org/10.1038/nature13777
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13777
This article is cited by
-
Plant-based biopharmaceutical engineering
Nature Reviews Bioengineering (2023)
-
A high-throughput single-particle imaging platform for antibody characterization and a novel competition assay for therapeutic antibodies
Scientific Reports (2023)
-
Evaluation of therapeutic PD-1 antibodies by an advanced single-molecule imaging system detecting human PD-1 microclusters
Nature Communications (2023)
-
Human Exposure to Bats, Rodents and Monkeys in Bangladesh
EcoHealth (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.