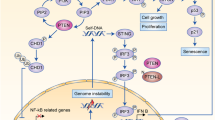
Abstract
Loss of the PTEN tumour suppressor is one of the most common oncogenic drivers across all cancer types1. PTEN is the major negative regulator of PI3K signalling. The PI3Kβ isoform has been shown to play an important role in PTEN-deficient tumours, but the mechanisms underlying the importance of PI3Kβ activity remain elusive. Here, using a syngeneic genetically engineered mouse model of invasive breast cancer driven by ablation of both Pten and Trp53 (which encodes p53), we show that genetic inactivation of PI3Kβ led to a robust anti-tumour immune response that abrogated tumour growth in syngeneic immunocompetent mice, but not in immunodeficient mice. Mechanistically, PI3Kβ inactivation in the PTEN-null setting led to reduced STAT3 signalling and increased the expression of immune stimulatory molecules, thereby promoting anti-tumour immune responses. Pharmacological PI3Kβ inhibition also elicited anti-tumour immunity and synergized with immunotherapy to inhibit tumour growth. Mice with complete responses to the combined treatment displayed immune memory and rejected tumours upon re-challenge. Our findings demonstrate a molecular mechanism linking PTEN loss and STAT3 activation in cancer and suggest that PI3Kβ controls immune escape in PTEN-null tumours, providing a rationale for combining PI3Kβ inhibitors with immunotherapy for the treatment of PTEN-deficient breast cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Transcriptomic data for bulk RNA-seq and scRNA-seq have been deposited at the Gene Expression Omnibus (GEO) under accession numbers GSE225064 and GSE224906. The scRNA-seq data has also been uploaded to the Single Cell Portal under accession number SCP2118. Source data are provided with this paper.
Code availability
Custom-made R code used for CyCIF analysis is available for download from Zenodo (https://doi.org/10.5281/zenodo.7675466). All R code used to generate figures in the manuscript can be made available upon reasonable request.
References
Lawrence, M. S. et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 505, 495–501 (2014).
Dong, Y. et al. PTEN functions as a melanoma tumor suppressor by promoting host immune response. Oncogene 33, 4632–4642 (2014).
Li, S. et al. The tumor suppressor PTEN has a critical role in antiviral innate immunity. Nat. Immunol. 17, 241–249 (2016).
George, S. et al. Loss of PTEN is associated with resistance to anti-PD-1 checkpoint blockade therapy in metastatic uterine leiomyosarcoma. Immunity 46, 197–204 (2017).
Parsa, A. T. et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 13, 84–88 (2007).
Peng, W. et al. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov. 6, 202–216 (2016).
Jia, S. et al. Essential roles of PI(3)K-p110β in cell growth, metabolism and tumorigenesis. Nature 454, 776–779 (2008).
Ni, J. et al. Functional characterization of an isoform-selective inhibitor of PI3K-p110β as a potential anticancer agent. Cancer Discov. 2, 425–433 (2012).
Wee, S. et al. PTEN-deficient cancers depend on PIK3CB. Proc. Natl Acad. Sci. USA 105, 13057–13062 (2008).
Wang, Q. et al. Spatially distinct roles of class Ia PI3K isoforms in the development and maintenance of PTEN hamartoma tumor syndrome. Genes Dev. 27, 1568–1580 (2013).
Network, C. G. A. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012).
Szabo, P. A. et al. Single-cell transcriptomics of human T cells reveals tissue and activation signatures in health and disease. Nat. Commun. 10, 4706–4716 (2019).
Zemmour, D. et al. Single-cell gene expression reveals a landscape of regulatory T cell phenotypes shaped by the TCR. Nat. Immunol. 19, 291–301 (2018).
Fu, C. & Jiang, A. Dendritic cells and CD8 T cell immunity in tumor microenvironment. Front. Immunol. 9, 3059 (2018).
Azizi, E. et al. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell 174, 1293–1308.e1236 (2018).
Schridde, A. et al. Tissue-specific differentiation of colonic macrophages requires TGFβ receptor-mediated signaling. Mucosal Immunol. 10, 1387–1399 (2017).
Tuit, S. et al. Transcriptional signature derived from murine tumor-associated macrophages correlates with poor outcome in breast cancer patients. Cell Rep. 29, 1221–1235.e1225 (2019).
Spranger, S. et al. Up-regulation of PD-L1, IDO, and Tregs in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci. Transl. Med. 5, 200ra116 (2013).
Iwabuchi, R. et al. Introduction of human Flt3-L and GM-CSF into humanized mice enhances the reconstitution and maturation of myeloid dendritic cells and the development of Foxp3+CD4+ T cells. Front. Immunol. 9, 1042 (2018).
Menezes, S. et al. The heterogeneity of Ly6Chi monocytes controls their differentiation into iNOS+ macrophages or monocyte-derived dendritic cells. Immunity 45, 1205–1218 (2016).
Yu, H., Pardoll, D. & Jove, R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer 9, 798–809 (2009).
Lien, E. C., Dibble, C. C. & Toker, A. PI3K signaling in cancer: beyond AKT. Curr. Opin. Cell Biol. 45, 62–71 (2017).
Guryanova, OlgaA. et al. Nonreceptor tyrosine kinase BMX maintains self-renewal and tumorigenic potential of glioblastoma stem cells by activating STAT3. Cancer Cell 19, 498–511 (2011).
Hart, J. R., Liao, L., Yates, J. R. & Vogt, P. K. Essential role of Stat3 in PI3K-induced oncogenic transformation. Proc. Natl Acad. Sci. USA 108, 13247–13252 (2011).
Liu, F. et al. Discovery of a selective irreversible BMX inhibitor for prostate cancer. ACS Chem. Biol. 8, 1423–1428 (2013).
Horn, L. A., Fousek, K. & Palena, C. Tumor plasticity and resistance to immunotherapy. Trends Cancer 6, 432–441 (2020).
Spranger, S., Bao, R. & Gajewski, T. F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 523, 231–235 (2015).
Tauriello, D. V. F. et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554, 538–543 (2018).
Sopasakis, V. R. et al. Specific roles of the p110α isoform of phosphatidylinsositol 3-kinase in hepatic insulin signaling and metabolic regulation. Cell Metab. 11, 220–230 (2010).
Schmit, F. et al. PI3K isoform dependence of PTEN-deficient tumors can be altered by the genetic context. Proc. Natl Acad. Sci. USA 111, 6395–6400 (2014).
Wang, Q. et al. PI3K-p110α mediates resistance to HER2-targeted therapy in HER2+, PTEN-deficient breast cancers. Oncogene 35, 3607–3612 (2016).
Liu, P. et al. Oncogenic PIK3CA-driven mammary tumors frequently recur via PI3K pathway-dependent and PI3K pathway-independent mechanisms. Nat. Med. 17, 1116–1120 (2011).
Wang, Q. et al. STING agonism reprograms tumor-associated macrophages and overcomes resistance to PARP inhibition in BRCA1-deficient models of breast cancer. Nat. Commun. 13, 3022 (2022).
Minami, A., Nakanishi, A., Ogura, Y., Kitagishi, Y. & Matsuda, S. Connection between tumor suppressor BRCA1 and PTEN in damaged DNA repair. Front. Oncol. 4, 318 (2014).
Halbrook, C. J. et al. Macrophage-released pyrimidines inhibit gemcitabine therapy in pancreatic cancer. Cell Metab. 29, 1390–1399.e1396 (2019).
Olson, O. C., Kim, H., Quail, D. F., Foley, E. A. & Joyce, J. A. Tumor-associated macrophages suppress the cytotoxic activity of antimitotic agents. Cell Rep. 19, 101–113 (2017).
Toso, A. et al. Enhancing chemotherapy efficacy in Pten-deficient prostate tumors by activating the senescence-associated antitumor immunity. Cell Rep. 9, 75–89 (2014).
Noh, K. H. et al. Activation of Akt as a mechanism for tumor immune evasion. Mol. Ther. 17, 439–447 (2009).
Lee, H. et al. A requirement of STAT3 DNA binding precludes Th-1 immunostimulatory gene expression by NF-κB in tumors. Cancer Res. 71, 3772–3780 (2011).
Kaneda, M. M. et al. PI3Kγ is a molecular switch that controls immune suppression. Nature 539, 437–442 (2016).
Kalinsky, K. et al. Ipatasertib in patients with tumors with AKT mutations: results from the NCI-MATCH ECOG-ACRIN Trial (EAY131) sub-protocol Z1K. Eur. J. Cancer 174, S8–S9 (2022).
Juric, D. et al. Phosphatidylinositol 3-kinase α-selective inhibition with alpelisib (BYL719) in PIK3CA-altered solid tumors: results from the first-in-human study. J. Clin. Oncol. 36, 1291–1299 (2018).
Juric, D. et al. Convergent loss of PTEN leads to clinical resistance to a PI(3)Kα inhibitor. Nature 518, 240–244 (2015).
Choudhury, A. D. et al. A phase i study investigating AZD8186, a potent and selective inhibitor of PI3Kβ/δ, in patients with advanced solid tumors. Clin. Cancer Res. 28, 2257–2269 (2022).
Dumbrava, E. E. et al. Phase I/II study of the selective PI3Kβ inhibitor GSK2636771 in combination with pembrolizumab in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) and PTEN loss. J. Clin. Oncol. 40, 5052–5052 (2022).
Jung, M. et al. SO-10: an open-label, multi-centre, phase Ib/II study of PI3Kβ selective inhibitor GSK2636771 administered in combination with paclitaxel in patients with advanced gastric cancer having alterations in PI3K/Akt pathway. Ann. Oncol. 32, S206 (2021).
Mateo, J. et al. A first-time-in-human study of GSK2636771, a phosphoinositide 3 kinase beta-selective inhibitor, in patients with advanced solid tumors. Clin. Cancer Res. 23, 5981–5992 (2017).
Lu, X. et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature 543, 728–732 (2017).
Simond, A. M., Rao, T., Zuo, D., Zhao, J. J. & Muller, W. J. ErbB2-positive mammary tumors can escape PI3K-p110α loss through downregulation of the Pten tumor suppressor. Oncogene 36, 6059–6066 (2017).
Reardon, D. A. et al. Glioblastoma eradication following immune checkpoint blockade in an orthotopic, immunocompetent model. Cancer Immunol. Res. 4, 124–135 (2016).
Palechor-Ceron, N. et al. Radiation induces diffusible feeder cell factor(s) that cooperate with ROCK inhibitor to conditionally reprogram and immortalize epithelial cells. Am. J. Pathol. 183, 1862–1870 (2013).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Lin, J.-R. et al. Highly multiplexed immunofluorescence imaging of human tissues and tumors using t-CyCIF and conventional optical microscopes. eLife 7, e31657 (2018).
Gierahn, T. M. et al. Seq-Well: portable, low-cost RNA sequencing of single cells at high throughput. Nat. Methods 14, 395–398 (2017).
Hughes, T. K. et al. Highly efficient, massively-parallel single-cell RNA-seq reveals cellular states and molecular features of human skin pathology. Preprint at bioRxiv https://doi.org/10.1101/689273 (2019).
Macosko, E. Z. et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Goel, S. et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 548, 471–475 (2017).
Krämer, A., Green, J., Pollard, J. & Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 30, 523–530 (2014).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Cerami, E. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 (2012).
Gao, J. et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 6, e2004088 (2013).
Acknowledgements
The authors thank J. Agudo, R. T. Bronson, L. Ding, M. Kearns, S. Lazo, B. Li, Z. Lin, H. Lu, J. Ni, C. Olson and J. Brugge for experimental assistance and scientific discussions; the laboratories of S. Santagata and P. Sorger and members of the Laboratory of Systems Pharmacology for assistance with tCyCIF; and E. Cahn and the Breast Cancer Advocacy Group (BCAG) at the Dana-Farber/Harvard Cancer Center (DF/HCC) for discussions on patient issues and needs. The authors thank the Dale Family Foundation for their charitable contributions; the DF/HCC Breast SPORE: Specialized Program of Research Excellence (SPORE), an NCI-funded programme, grant 1P50CA168504, for their contributions in the collection and distribution of biospecimens used for establishing PDX models. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health/NCI. This work was supported in part by grants from The Susan G. Komen Foundation PDF16376814 (J.S.B.), Friends of Dana-Farber Cancer Institute (J.S.B.), Cancer Research Institute (Qiwei Wang), Friends of Dana-Farber Cancer Institute (Qiwei Wang), Department of Defense Breast Cancer Research Program Breakthrough Award HT9425-23-1-0026 (Qiwei Wang), Terri Brodeur Breast Cancer Foundation (S.K.), Program Project Grant in Breast and Gynecologic Cancers from DFCI Susan F. Smith Center for Women’s Cancers (G.J.F.), Pew-Stewart Scholars Program for Cancer Research (A.K.S.), Sloan Fellowship in Chemistry (A.K.S.), Breast Cancer Research Foundation (J.J.Z.), and NIH P50 CA101942 (G.J.F.), P50CA206963 (G.J.F.), 1DP2GM119419 (A.K.S.), 1U54CA217377 (A.K.S.), P50 CA168504 (T.M.R. and J.J.Z), CA231945 (T.M.R.) and CA210057 (J.J.Z).
Author information
Authors and Affiliations
Contributions
J.S.B., T.M.R. and J.J.Z. designed and guided the direction of the project. J.S.B., Qiwei Wang and J.J.Z. contributed to the design, analysis and interpretation of all experiments. J.S.B. performed animal dissections, tumour and tissue manipulations and various in vitro experiments, including tissue culturing, drug treatments, virus production and transductions, western blotting, RNA isolation and qPCR, and analysed flow cytometry, CyCIF, bulk RNA-seq and scRNA-seq data. Qiwei Wang designed, performed and analysed flow cytometry and co-culture experiments, and performed tumour and tissue manipulations and various in vitro experiments, including tissue culturing, drug treatments and transfections, CRISPR–Cas 9 editing, western blotting, RNA isolation and qPCR. Qi Wang and J.J.Z. conceived and developed PP, PPA and PPB GEM models of breast tumours and contributed to the design of in vivo efficacy studies. M.R. and S.P. designed, performed, analysed and interpreted scRNA-seq experiments. W.W. and K.A.-H. contributed to the design and performed in vivo drug treatments, performed tumour measurements, contributed to animal health monitoring, performed animal dissections and tumour and tissue manipulations, and performed various in vitro experiments, including tissue culturing, drug treatments, immunohistochemistry, western blotting, RNA isolation, qPCR and ELISA. R.F. contributed to tumour measurements in vivo, and performed numerous in vitro experiments, including cloning, western blotting, RNA isolation, qPCR and ELISA. S.K. designed, performed and analysed CyCIF experiments. Q.Z. and S.T. designed and performed the PPB-mPI3Kβ rescue experiments. G.K.G. designed and performed pilot immune profiling experiments by CyTOF used to guide the design of future immune profiling experiments by flow cytometry. S.X. and H.G. performed and contributed to the analysis of gene expression experiments by RNA-seq. X.G. performed in vitro drug response experiments, western blots, qPCR and ELISA. T.V. and T.J. performed tumour transplantations, in vivo drug treatments and tumour measurements, and contributed to animal health monitoring. G.J.F., H.-J.K. and A.K.S. contributed with key reagents, protocols and scientific discussions. J.S.B. and J.J.Z. wrote the paper. J.S.B., Qiwei Wang, M.R., G.K.G., G.J.F., H.-J.K., A.K.S., T.M.R. and J.J.Z. reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.S.B. is a scientific consultant for Geode Therapeutics Inc. J.S.B., G.J.F., T.M.R. and J.J.Z. are co-inventors of DFCI 2180.001 (DFS-166.25) related to this work. Qiwei Wang is a scientific consultant for Crimson Biopharm Inc. Qi Wang is currently an employee at Geode Therapeutics Inc. S.P. is currently an employee at Takeda Pharmaceuticals. S.X. is currently an employee at HiFiBiO Therapeutics. T.V. is currently an employee at MarvelBiome Inc. G.J.F. has patents and pending royalties on the PD-1–PD-L1 pathway from Roche, Merck MSD, Bristol-Myers-Squibb, Merck KGA, Boehringer-Ingelheim, AstraZeneca, Dako, Leica, Mayo Clinic, Eli Lilly and Novartis. G.J.F. has served on advisory boards for Roche, Bristol-Myers-Squibb, Xios, Origimed, Triursus, iTeos, NextPoint, IgM, Jubilant, Trillium, IOME, Geode, Bright Peak, and GV20. G.J.F. has equity in Nextpoint, Triursus, Xios, iTeos, IgM, Trillium, Invaria, Geode and GV20. H.-J.K. is currently an employee at Genentech. A.K.S. has received compensation for consulting and/or SAB membership from Merck, Honeycomb Biotechnologies, Cellarity, Repertoire Immune Medicines, Hovione, Third Rock Ventures, Ochre Bio, FL82, Empress Therapeutics, Relation Therapeutics, Senda Biosciences, IntrECate biotherapeutics, Santa Ana Bio, and Dahlia Biosciences unrelated to this work. T.M.R. is a SAB member for Shiftbio and K2B Therapeutics and is a co-founder of Geode Therapeutics Inc. J.J.Z. is a co-founder and board director of Crimson Biotech Inc. and Geode Therapeutics Inc. All other authors declare no competing interests.
Peer review
Peer review information
Nature thanks Emilio Hirsch, Maurizio Scaltriti and Judith Varner for their contribution to the peer review of this work. Peer review reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Characterization of breast cancer GEM models.
a, H&E staining of primary PP (n = 4), PPA (n = 3) and PPB (n = 4) tumors. Representative images shown. b, Genetic alterations in PTEN and TP53 in patient samples of PAM50 basal breast cancer from The Cancer Genome Atlas (TCGA). c–e, Characterization of PP, PPA and PPB primary tumor cells by western blotting (c) and RNA-Seq (d; n = 5 biological replicates for MMEC; n = 3 biological replicates for PP, PPA and PPB), compared to positive controls for PTEN (MCF7 and HCC1954) and Trp53 (mouse mammary epithelial cells; MMEC). Data in (d) presented as mean values and s.d. e, Tumor cells were treated with 1.0 µM pan-PI3K inhibitor (BKM-120), PI3Kα inhibitor (BYL719) or PI3Kβ inhibitor (AZD6482) for one hour in complete growth media containing 10% FBS. Whole-cell lysates were analyzed by western blotting. Immunoblot representative of two independent experiments. f, Western blot analysis of primary tumor cells. Immunoblot representative of three independent experiments. g, H&E staining of transplanted tumors or remaining tumor bed from nude and FVB mice. Scale bars = 100 μm. Representative images shown (nude; PP, n = 8 tumors; PPA and PPB, n = 4 tumors; FVB, n = 8 tumors per group). h, Tumor-free survival of Rag1+/− (n = 4 mice) and Rag1−/− (n = 8 mice) FVB mice injected with PPB tumor cells. Statistical analysis by Log-rank (Mantel-Cox) test. i–k, PPB tumor cells were stably transfected with a vector control or mouse PI3Kβ. i, Western blot analysis. j, Tumor growth in immunocompetent syngeneic FVB mice (n = 5 tumors per group). Data presented as mean values and s.e.m. k, Tumors after necropsy. l, PPB tumor volume after transplanting into FVB mice treated with or without CD4 or CD8 depleting antibodies (n = 10 tumors per group). Inset shows magnified view of early time points. Data presented as mean values and s.e.m. Apparent molecular weights in kDa are indicated for immunoblots.
Extended Data Fig. 2 PPB tumor cells exhibit reduced levels of proliferation in vitro and in vivo.
a, Cell proliferation in vitro under reduced serum/nutrient conditions. Data shown as mean and s.d. (n = 6 biological replicates per condition). b–c, Analysis of GFP-tagged PP, PPA and PPB tumors harvested five days after tumor cell transplantation into nude mice. Representative sections (b) and quantification (c) are shown (n = 6 tumors for PP and PPB; n = 3 tumors for PPA). GFP was added via lentiviral transduction in vitro prior to transplantations. Scale bars: 100 μm (left) and 50 μm (right). Box plots represent median and inter-quartile range, and min-to-max error bars (whiskers). For comparison of multiple mean values (a and c), one-way ANOVA followed by Tukey’s multiple comparisons tests.
Extended Data Fig. 3 Single-cell RNA-Seq analysis of immune infiltrate in PTEN/p53-null breast tumors with catalytic isoform-specific PI3K deletion.
Single-cell RNA-Seq (scRNA-Seq) analysis of immune infiltrate from PP, PPA and PPB tumors (n = 3 mice per group). Tumors were harvested and dissociated into single cells, followed by FACS sorting for viable immune (CD45+) cells prior to scRNA-Seq analysis via Seq-Well technology. a, Heatmap showing gene expression for top 10% differentially-expressed genes (DEGs) for each cell type cluster. Selected genes for each cluster are indicated on the right. Genotype of the tumor of origin is indicated on top. b, tSNE of all CD45+ cells analyzed denoting experimental replicate and cell type. c, Genotype representation per cell type. Complete list of DEGs per cell type is available in the source data linked to this article.
Extended Data Fig. 4 Single-cell RNA-Seq analysis of monocytes/macrophages cluster in PTEN/p53-null breast tumors with catalytic isoform-specific PI3K deletion.
Single-cell RNA-Seq analysis of monocytes/macrophages (MoMϕ) cluster from PP, PPA and PPB tumors (n = 3 mice per group). a, Heatmap showing gene expression for top 10% differentially-expressed genes (DEGs) for each MoMϕ sub-cluster. Selected genes are indicated on the right. Genotype of the tumor of origin is indicated on top. b, tSNE of all MoMϕ analyzed denoting sub-cluster. c, MoMϕ sub-cluster representation per genotype. d, Genotype representation per MoMϕ sub-cluster. e, GSEA of MoMϕ sub-clusters. Statistical significance of enrichment scores (p-values) were calculated by GSEA using a phenotype-based permutation test. Complete list of DEGs per MoMϕ sub-cluster is available in the source data linked to this article.
Extended Data Fig. 5 Enhanced immune cell activation in PPB tumors.
a–g, Flow cytometry analysis of PP, PPA and PPB tumors harvested five days after tumor cell transplantation into FVB mice (n = 10 tumors per group). Box plots represent median and inter-quartile range, and min-to-max error bars (whiskers). Representative gating profiles to demonstrate that myeloid-derived IFNɣ is primarily produced by inflammatory monocytes are shown on (f). h, PP, PPA and PPB tumor cells were treated with 100 ng/mL IFNɣ in vitro for 48 h. MHC class I (MHC-I) expression was analyzed by flow cytometry (n = 3 biological replicates per condition). Data shown as median and min-to-max error bars. i, PP, PPA and PPB tumor cells were treated with IFNɣ in vitro for 72 h. Cell viability was analyzed by CellTiter-Glo. Data shown as mean values and s.d. (n = 4 biological replicates per condition). For comparison of two means (h), unpaired, two-tail t-test with Welch’s correction assuming unequal variance. For multiple comparisons (a–e, g and i), one-way ANOVA followed by Tukey’s multiple comparisons tests. Flow cytometry gating profiles available on Supplementary Fig. 1.
Extended Data Fig. 6 PPB tumor cells enhance immune cell activation ex vivo, and show enhanced inflammatory signaling and reduced STAT3 pathway activation.
a–d, Flow cytometry analyses of immune cells from co-culture experiments ex vivo. a, Analysis of bone marrow-derived cells (BMCs) co-cultured with tumor cells (n = 5 biological replicates per group). b, Analysis of bone marrow-derived dendritic cells (BMDCs) co-cultured with tumor cells (n = 4 biological replicates per group). c, Analysis of CD8+ T-cells co-cultured with tumor cells (n = 4 biological replicates per group). d, Analysis of CD8+ T-cells co-cultured with dendritic cells (DCs) that had previously been co-cultured with tumor cells (n = 4 biological replicates per group). Box plots represent median and inter-quartile range, and min-to-max error bars (whiskers). Flow cytometry gating profiles available on Supplementary Fig. 2. e, Heatmap of gene expression for immune-related genes in primary tumor cells (n = 3 biological replicates per group). f, Analysis by Q-PCR (left) and ELISA (right) of GM-CSF expression by PP and PPB cells. Data presented as mean values and s.d. (n = 3 biological replicates per group). g, Analysis by Q-PCR (left; n = 4 biological replicates per group) and ELISA (right; n = 3 biological replicates per group) of IL-6 expression by PP and PPB cells. Data presented as mean values and s.d. h–i, Immunofluorescence analysis of PP, PPA and PPB tumors harvested five days after tumor cell transplantation into FVB mice, including representative sections (h; n = 3 tumors per group) and quantification of STAT3Y705 phosphorylation in tumor cells as assessed by fluorescence intensity (i). Results correspond to further analysis of samples in Fig. 2a. Tumor cells show positive P-AKT staining (red color). Blue and red-lined arrows point to examples of PP and PPA tumor cells with high P-STAT3. Green-lined arrows point to PPB tumor cell clusters highlighted with white dashed line. For comparison of two means (a–g), unpaired, two-tail t-test with Welch’s correction assuming unequal variance. OD, optical density; TNF, tumor necrosis factor; TLR, toll-like receptor.
Extended Data Fig. 7 The PI3Kβ-STAT3 pathway mediates immune suppressive signaling in PTEN/p53-null breast tumor cells.
a–d, Analysis of PPB cells stably expressing an empty vector control (vector) or constitutively-active STAT3A661C, N663C (STAT3-CA) by western blotting (a), gene expression (b; n = 3 biological replicates per group), GSEA (c; n = 3 biological replicates per group), and ELISA (d; n = 3 biological replicates per group). e, Flow cytometry analysis of dendritic cells (DCs) co-cultured with tumor cells (n = 4 biological replicates per group). f–i, Analysis of PP cells transduced with shRNA against GFP (shGFP) or Stat3 (shStat3; two independent constructs) by western blotting (f), gene expression (g; n = 3 biological replicates per group), GSEA (h; n = 3 biological replicates per group), and ELISA (i; n = 3 per group). j, Flow cytometry analysis of DCs co-cultured with tumor cells (n = 8 biological replicates per group). k, Tumor volume after transplantation into immunodeficient mice (nude; n = 10 tumors per group) or syngeneic immunocompetent mice (FVB; n = 8 tumors for PP-shGFP and PP-shStat3(1); n = 6 tumors for PP-shStat3(2)). Data presented as mean values and s.e.m. l, Flow cytometry analysis of immune infiltrate after tumor transplantation into FVB mice. Apparent molecular weights in kDa are indicated for immunoblots. Data on (b), (d), (g) and (i) are presented as mean values and s.d. Box plots represent median and inter-quartile range, and min-to-max error bars (whiskers). For comparison of two means (b, d–e), unpaired, two-tail t-test with Welch’s correction assuming unequal variance. For multiple comparisons (g,i,j and l), one-way ANOVA followed by Tukey’s multiple comparisons tests. Flow cytometry gating profiles available on Supplementary Fig. 4. CR, complete regression.
Extended Data Fig. 8 PI3Kβ regulates STAT3 signaling via TEC Family Kinase BMX.
a, Western blot analysis of PP cells treated with AZD6482 or DMSO as a control in complete growth media or under serum/nutrient starvation conditions. Immunoblot representative of two independent experiments. b–c, Analysis of PP cells treated with AZD6482 under serum/nutrient starvation by gene expression (b; n = 3 biological replicates per condition), and Q-PCR (GM-CSF, n = 3 biological replicates per group; IL-6, n = 4 biological replicates per group) and ELISA (GM-CSF, n = 4 biological replicates per group; IL-6, n = 6 biological replicates per group) (c). For comparison of two means, unpaired, two-tail t-test with Welch’s correction assuming unequal variance. Data in (c) presented as mean values and s.d. d, PP tumor-bearing nude mice were treated with a vehicle control or AZD6482. RNA from isolated tumor cells was analyzed by RNA-Seq. Results from GSEA for AZD6482 compared to vehicle are shown (n = 3 tumors per group). e, Western blot analysis of PTEN-deficient human breast cancer cells treated with AZD6482 or DMSO as a control under reduced nutrient/serum conditions. Immunoblots representative of two biological replicates per condition. f, Immunohistochemistry analysis of HCC70 xenografts from nude mice treated with AZD6482 or a vehicle control. Representative images shown (n = 5 tumors per condition). Scale bars = 100 μm and 50 μm (inset). g–i, Western blot analysis of PP cells treated with the AKT inhibitor MK2206 (g), the TEC Kinase family inhibitor LFM-A13 (h) or the BMX inhibitor BMX-IN-1 (i) under serum/nutrient starvation conditions. Immunoblots representative of two independent experiments performed in duplicate. j, Molecular mechanisms working model. k, Summary of PDX models denoting PTEN genomic status and PTEN protein levels as assessed by IHC. Representative IHC sections are shown (n = 2 tumors per model). Scale bars, 300 μm. Apparent molecular weights in kDa are indicated for immunoblots.
Extended Data Fig. 9 PD-1 blockade potentiates anti-tumor immune response induced by PI3Kβ inhibition in PTEN-null mouse mammary tumors.
a–b, Flow cytometry analysis of tumor cells and immune infiltrate from PP (a) or HER2/Neu+ (b) tumors harvested from FVB mice treated with AZD6482 or a vehicle control (n = 10 tumors per condition). c, H&E stain of representative tumor samples from mice (Exp. 1) treated with vehicle (n = 8) or combined AZD6482 and PD-1 blockade (αPD-1), including cases showing complete response (CR; n = 3) and delayed progressive disease (PD; n = 3). Scale bars = 100 μm. d–f, Flow cytometry analysis of tumor cells and immune infiltrate from PP tumor-bearing FVB mice treated with AZD6482 or αPD-1 as single agents or in combination (n = 10 tumors per group). Box plots represent median and inter-quartile range, and min-to-max error bars (whiskers). For comparison of two means (a–b), unpaired, two-tail t-test with Welch’s correction assuming unequal variance. For multiple comparisons (d–f), one-way ANOVA followed by Tukey’s multiple comparisons tests. g–h, RNA from bulk PP tumor fragments was analyzed by RNA-Seq (n = 6 per group). Results from GSEA compared to the vehicle control (g) and heatmap denoting expression of immune-related genes (h) are shown. Flow cytometry gating profiles available on Supplementary Figs. 5 and 6. MFI, mean fluorescence intensity; IFN, interferon; NES, normalized enrichment score; PRRs, pattern recognition receptors.
Extended Data Fig. 10 Mice with complete response to combined PI3Kβ inhibition and PD-1 blockade exhibit robust anti-tumor immunity to tumor re-challenge.
a–c, Flow cytometry analysis of tumor immune infiltrate (a; n = 12 tumors for naïve; n = 10 tumors for re-challenged), draining lymph nodes (b; n = 12 lymph nodes for naïve; n = 10 lymph nodes for re-challenged) and spleens (c; n = 6 spleens for naïve; n = 5 spleens for re-challenged) from age-matched FVB mice challenged for the first time (naïve; n = 6 mice) and in mice with complete response to combined treatment and challenged for a second time (re-challenged; n = 5 mice). Tissues were harvested two weeks after re-challenging. Box plots represent median and inter-quartile range, and min-to-max error bars (whiskers). For comparison of two means, unpaired, two-tail t-test with Welch’s correction assuming unequal variance. Flow cytometry gating profiles available on Supplementary Fig. 7. MFI, mean fluorescence intensity; Tregs, regulatory T-cells.
Extended Data Fig. 11 PTEN loss sensitizes tumors to enhancement of anti-tumor immune response by pharmacological PI3Kβ-specific inhibition.
a, Western blot analysis of RCT-E302 mouse mammary tumor cells with deleted Pten (RCT-E302-sgPten) and parental RCT-E302 cells. b–c, Flow cytometry analysis of tumor cells and immune infiltrate from RCT-E302-sgPten (b) or RCT-E302 (c) tumors harvested from FVB mice treated with AZD6482 or a vehicle control (n = 10 tumors per group). d, Western blot analysis of BPP (Brca1/Trp53/Pten triple-null) and BP (Brca1/Trp53 double-null) mouse mammary tumor cells. e–f, Flow cytometry analysis of tumor cells and immune infiltrate from BPP (e) or BP (f) tumors harvested from FVB mice treated with AZD6482 or a vehicle control (n = 10 tumors per group). Apparent molecular weights in kDa are indicated for immunoblots. For comparison of two means (b–c and e–f), unpaired, two-tail t-test with Welch’s correction assuming unequal variance. Flow cytometry gating profiles available on Supplementary Fig. 8.
Extended Data Fig. 12 Combined PI3Kβ inhibition and immunotherapy inhibit tumor growth in mouse models of PTEN-null breast cancer.
a–b, Tumor-bearing mice were treated with AZD6482 or a monoclonal antibody against mouse PD-1 (αPD-1) alone or in combination, as shown. Tumor growth curves (left panels) and tumor growth inhibition (TGI; right panels) are shown (n = 8 tumors for AZD6482 in (a); n = 10 tumors for other conditions in (a) and (b)). c, Western blot analysis of 4T1 mouse mammary tumor cells with deleted Pten (4T1-sgPten) and parental 4T1 cells. Image is representative of two independent immunoblots. Apparent molecular weights in kDa are indicated. d, Ratio of M2-like polarized (CD206High MHC-II-) to M1-like polarized (CD206- MHC-IIHigh) macrophages (CD11b+ F4/80+) as determined by flow cytometry (n = 10 tumors per group). e, 4T1-sgPten tumor-bearing mice were treated with AZD6482, αPD-1 or the STING agonist MSA2 alone or in combination. Tumor growth curves (left panels) and TGI (right panels) are each shown as two separate plots for clarity, with all plots including the same vehicle and AZD6482 groups for easier comparison (n = 8 tumors for MSA; n = 10 tumors for other conditions). Data for tumor growth curves presented as mean values and s.e.m. Box plots represent median and inter-quartile range, and min-to-max error bars (whiskers). For comparison of multiple mean values (a–b and d–e), one-way ANOVA followed by Tukey’s multiple comparisons tests. Statistical analysis on (e) was performed including all groups in the comparisons, with results shown in two different graphs for easier visual assessment. Flow cytometry gating profiles are available on Supplementary Fig. 9.
Supplementary information
Supplementary Information
Supplementary Figs. 1–25
Supplementary Tables
Supplementary Tables 1–14
Supplementary Tables 15–17
Tables listing antibodies used for CyCIF, flow cytometry and western blots.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bergholz, J.S., Wang, Q., Wang, Q. et al. PI3Kβ controls immune evasion in PTEN-deficient breast tumours. Nature 617, 139–146 (2023). https://doi.org/10.1038/s41586-023-05940-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-05940-w
This article is cited by
-
Nanoparticles in tumor microenvironment remodeling and cancer immunotherapy
Journal of Hematology & Oncology (2024)
-
A novel pan-PI3K inhibitor KTC1101 synergizes with anti-PD-1 therapy by targeting tumor suppression and immune activation
Molecular Cancer (2024)
-
Enzyme lights dual fires to promote cancer
Nature (2023)
-
PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer
Molecular Cancer (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.