Abstract
Medulloblastoma (MB) comprises a group of heterogeneous paediatric embryonal neoplasms of the hindbrain with strong links to early development of the hindbrain1,2,3,4. Mutations that activate Sonic hedgehog signalling lead to Sonic hedgehog MB in the upper rhombic lip (RL) granule cell lineage5,6,7,8. By contrast, mutations that activate WNT signalling lead to WNT MB in the lower RL9,10. However, little is known about the more commonly occurring group 4 (G4) MB, which is thought to arise in the unipolar brush cell lineage3,4. Here we demonstrate that somatic mutations that cause G4 MB converge on the core binding factor alpha (CBFA) complex and mutually exclusive alterations that affect CBFA2T2, CBFA2T3, PRDM6, UTX and OTX2. CBFA2T2 is expressed early in the progenitor cells of the cerebellar RL subventricular zone in Homo sapiens, and G4 MB transcriptionally resembles these progenitors but are stalled in developmental time. Knockdown of OTX2 in model systems relieves this differentiation blockade, which allows MB cells to spontaneously proceed along normal developmental differentiation trajectories. The specific nature of the split human RL, which is destined to generate most of the neurons in the human brain, and its high level of susceptible EOMES+KI67+ unipolar brush cell progenitor cells probably predisposes our species to the development of G4 MB.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The bulk and scRNA-seq data generated from MB tumour samples in this study have been deposited in the European Genome-Phenome Archive (EGA) database under the accession code EGAS00001005826. The bulk and scRNA-seq data generated from the G3 MB cell lines HDMB03 and MB3W1 in this study have been deposited in the Gene Expression Omnibus (GEO) database under the access codes GSE189238 and GSE200791, respectively. The published MB bulk and scRNA-seq data referenced in this study are available in the EGA database under the accessions EGAD00001006305, EGAD00001004435, EGAD00001004958, EGAS00001003170 and EGAS00001003368. The referenced GTEx normal cerebellum RNA-seq controls were acquired from the NCBI public repository phs000424.v6.p1. The Affymetrix SNP 6.0 data referenced during the study are available in the GEO database under the accession GSE37385. The whole-genome sequencing data referenced during the study are available in the EGA database under the accessions EGAD00001003125 and EGAD00001004347. The Illumina 450k methylation data referenced during the study are available in the GEO database under the accession GSE85218. The G3 tumoursphere ChIP-seq data referenced during the study are available in the GEO database under the accession GSE132269. There were multiple databases used for annotation and filtering referenced in this study. These include the Exome Aggregation Consortium/gnomAD (https://gnomad.broadinstitute.org/downloads), the NHLBI-ESP project (https://esp.gs.washington.edu/drupal/), the Kaviar Genomic Variant Database (http://db.systemsbiology.net/kaviar/#:~:text=Kaviar%20Genomic%20Variant%20Database%20%7C%20SNP,and%20frequency%20of%20observed%20variants.), the Haplotype Reference Consortium (http://www.haplotype-reference-consortium.org/), the Greater Middle East Variome (http://igm.ucsd.edu/gme/), the Brazilian Genomic Variants Database (http://abraom.ib.usp.br/), RADAR (http://rnaedit.com/), GENCODE (v.19) (https://www.gencodegenes.org/human/release_19.html), the hs37d5 reference genome (https://ftp-trace.ncbi.nih.gov/1000genomes/ftp/technical/reference/phase2_reference_assembly_sequence/), ERCC spike-in sequence (https://www.encodeproject.org/files/ENCFF908UQN/) and Caltech profile 3 spike-in sequence (https://www.encodeproject.org/references/ENCSR193ZXE/). snRNA-seq data from the developing human cerebellum were obtained through correspondence from ref. 13 and are available through the Human Cell Atlas (https://www.covid19cellatlas.org/aldinger20), the UCSC Cell Browser (https://cbl-dev.cells.ucsc.edu) or from Database of Genotypes and Phenotypes (dbGaP) (accession number phs001908.v2.p1). Bulk RNA-seq data from the developing human cerebellum were obtained through correspondence from ref. 12 and are available through the dbGaP (accession number phs001908.v2.p1). Source data are provided with this paper.
Code availability
No custom code was used in this study. Only open-source algorithms were used, and their applications are detailed in the Methods. Further details on how these algorithms were used are available from the corresponding author upon request.
Change history
07 October 2022
In the version of this article initially published, the wrong year (2008) was provided for reference 37, which has been corrected to read 1968 in the HTML and PDF versions of the article.
29 November 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41586-022-05578-0
References
Northcott, P. A. et al. Medulloblastoma comprises four distinct molecular variants. J. Clin. Oncol. 29, 1408–1414 (2011).
Cavalli, F. M. G. et al. Intertumoral heterogeneity within medulloblastoma subgroups. Cancer Cell 31, 737–754.e6 (2017).
Vladoiu, M. C. et al. Childhood cerebellar tumors mirror conserved fetal transcriptional programs. Nature 572, 67–73 (2019).
Hovestadt, V. et al. Resolving medulloblastoma cellular architecture by single-cell genomics. Nature 572, 74–79 (2019).
Wechsler-Reya, R. J. & Scott, M. P. Control of neuronal precursor proliferation in the cerebellum by Sonic hedgehog. Neuron 22, 103–114 (1999).
Wallace, V. A. Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr. Biol. 9, 445–448 (1999).
Yang, Z.-J. et al. Medulloblastoma can be initiated by deletion of patched in lineage-restricted progenitors or stem cells. Cancer Cell 14, 135–145 (2008).
Oliver, T. G. et al. Loss of patched and disruption of granule cell development in a pre-neoplastic stage of medulloblastoma. Development 132, 2425–2439 (2005).
Gibson, P. et al. Subtypes of medulloblastoma have distinct developmental origins. Nature 468, 1095–1099 (2010).
Jessa, S. et al. Stalled developmental programs at the root of pediatric brain tumors. Nat. Genet. 51, 1702–1713 (2019).
Northcott, P. A. et al. The whole-genome landscape of medulloblastoma subtypes. Nature 547, 311–317 (2017).
Haldipur, P. et al. Spatiotemporal expansion of primary progenitor zones in the developing human cerebellum. Science 366, 454–460 (2019).
Aldinger, K. A. et al. Spatial and cell type transcriptional landscape of human cerebellar development. Nat. Neurosci. 24, 1163–1175 (2021).
Juraschka, K. & Taylor, M. D. Medulloblastoma in the age of molecular subgroups: a review. J. Neurosurg. Pediatr. 24, 353–363 (2019).
Tu, S. et al. Co-repressor CBFA2T2 regulates pluripotency and germline development. Nature 534, 387–390 (2016).
Nady, N. et al. ETO family protein Mtgr1 mediates Prdm14 functions in stem cell maintenance and primordial germ cell formation. eLife 4, e10150 (2015).
Tracey, L. J. et al. The pluripotency regulator PRDM14 requires hematopoietic regulator CBFA2T3 to initiate leukemia in mice. Mol. Cancer Res. 17, 1468–1479 (2019).
Baulies, A. et al. The transcription co-repressors MTG8 and MTG16 regulate exit of intestinal stem cells from their niche and differentiation into enterocyte vs secretory lineages. Gastroenterology 159, 1328–1341.e3 (2020).
Stadhouders, R. et al. Control of developmentally primed erythroid genes by combinatorial co-repressor actions. Nat. Commun. 6, 8893 (2015).
Northcott, P. A. et al. Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature 488, 49–56 (2012).
Waszak, S. M. et al. Germline elongator mutations in Sonic hedgehog medulloblastoma. Nature 580, 396–401 (2020).
Badodi, S. et al. Convergence of BMI1 and CHD7 on ERK signaling in medulloblastoma. Cell Rep. 21, 2772–2784 (2017).
Skowron, P. et al. The transcriptional landscape of Shh medulloblastoma. Nat. Commun. 12, 1749 (2021).
Parang, B. et al. The transcriptional corepressor MTGR1 regulates intestinal secretory lineage allocation. FASEB J. 29, 786–795 (2015).
Branon, T. C. et al. Efficient proximity labeling in living cells and organisms with TurboID. Nat. Biotechnol. 36, 880–887 (2018).
Forget, A. et al. Aberrant ERBB4–SRC signaling as a hallmark of group 4 medulloblastoma revealed by integrative phosphoproteomic profiling. Cancer Cell 34, 379–395.e7 (2018).
Linggi, B. & Carpenter, G. ErbB-4 s80 intracellular domain abrogates ETO2-dependent transcriptional repression. J. Biol. Chem. 281, 25373–25380 (2006).
Northcott, P. A. et al. Enhancer hijacking activates GFI1 family oncogenes in medulloblastoma. Nature 511, 428–434 (2014).
Haldipur, P. et al. Evidence of disrupted rhombic lip development in the pathogenesis of Dandy–Walker malformation. Acta Neuropathol. 142, 761–776 (2021).
Haldipur, P. et al. Expression of Sonic hedgehog during cell proliferation in the human cerebellum. Stem. Cells Dev. 21, 1059–1068 (2012).
Englund, C. Unipolar brush cells of the cerebellum are produced in the rhombic lip and migrate through developing white matter. J. Neurosci. 26, 9184–9195 (2006).
Leto, K. et al. Consensus paper: cerebellar development. Cerebellum 15, 789–828 (2016).
Stromecki, M. et al. Characterization of a novel OTX2-driven stem cell program in group 3 and group 4 medulloblastoma. Mol. Oncol. 12, 495–513 (2018).
Zagozewski, J. et al. An OTX2–PAX3 signaling axis regulates group 3 medulloblastoma cell fate. Nat. Commun. 11, 3627 (2020).
Brzustowicz, R. J. Cell rests in the region of the fourth ventricle. AMA Arch. Neurol. Psychiatry 67, 592 (1952).
Raaf, J. Relation of abnormal collections of cells in posterior medullary velum of cerebellum to origin of medulloblastoma. Arch. Neurol. Psychiatry 52, 163 (1944).
Rorke, L. B., Fogelson, M. H. & Riggs, H. E. Cerebellar heterotopia in infancy. Dev. Med. Child Neurol. 10, 644–650 (1968).
Yachnis, A. T., Rorke, L. B. & Trojanowski, J. Q. Cerebellar dysplasias in humans: development and possible relationship to glial and primitive neuroectodermal tumors of the cerebellar vermis. J. Neuropathol. Exp. Neurol. 53, 61–71 (1994).
Yang, J. & Zhang, Y. I-TASSER server: new development for protein structure and function predictions. Nucleic Acids Res. 43, W174–W181 (2015).
Mermel, C. H. et al. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol. 12, R41 (2011).
Canisius, S., Martens, J. W. M. & Wessels, L. F. A. A novel independence test for somatic alterations in cancer shows that biology drives mutual exclusivity but chance explains most co-occurrence. Genome Biol. 17, 261 (2016).
Finak, G. et al. MAST: a flexible statistical framework for assessing transcriptional changes and characterizing heterogeneity in single-cell RNA sequencing data. Genome Biol. 16, 278 (2015).
Ximerakis, M. et al. Single-cell transcriptomic profiling of the aging mouse brain. Nat. Neurosci. 22, 1696–1708 (2019).
Aibar, S. et al. SCENIC: single-cell regulatory network inference and clustering. Nat. Methods 14, 1083–1086 (2017).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Milde, T. et al. HD-MB03 is a novel group 3 medulloblastoma model demonstrating sensitivity to histone deacetylase inhibitor treatment. J. Neurooncol. 110, 335–348 (2012).
Dietl, S. et al. MB3W1 is an orthotopic xenograft model for anaplastic medulloblastoma displaying cancer stem cell- and group 3-properties. BMC Cancer 16, 115 (2016).
Choi, H. et al. SAINT: probabilistic scoring of affinity purification–mass spectrometry data. Nat. Methods 8, 70–73 (2011).
Teo, G. et al. SAINTexpress: improvements and additional features in significance analysis of INTeractome software. J. Proteomics 100, 37–43 (2014).
Chen, S., Zhou, Y., Chen, Y. & Gu, J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890 (2018).
Patro, R., Duggal, G., Love, M. I., Irizarry, R. A. & Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419 (2017).
Suzuki, H. et al. Recurrent noncoding U1 snRNA mutations drive cryptic splicing in SHH medulloblastoma. Nature 574, 707–711 (2019).
Auwera, G. A. et al. From FastQ data to high‐confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr. Protoc. Bioinformatics 43, 11.10.1–11.10.33 (2013).
Wang, K., Li, M. & Hakonarson, H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38, e164 (2010).
Lawrence, M. S. et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499, 214–218 (2013).
Mularoni, L., Sabarinathan, R., Deu-Pons, J., Gonzalez-Perez, A. & López-Bigas, N. OncodriveFML: a general framework to identify coding and non-coding regions with cancer driver mutations. Genome Biol. 17, 128 (2016).
Haas, B. J. et al. Accuracy assessment of fusion transcript detection via read-mapping and de novo fusion transcript assembly-based methods. Genome Biol. 20, 213 (2019).
Okonechnikov, K. et al. InFusion: advancing discovery of fusion genes and chimeric transcripts from deep RNA-sequencing data. PLoS ONE 11, e0167417 (2016).
Robertson, G. et al. De novo assembly and analysis of RNA-seq data. Nat. Methods 7, 909–912 (2010).
Zheng, G. X. Y. et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 8, 14049 (2017).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e29 (2021).
Hafemeister, C. & Satija, R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 20, 296 (2019).
Patel, A. P. et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344, 1396–1401 (2014).
Khazaei, S. et al. H3.3 G34W promotes growth and impedes differentiation of osteoblast-like mesenchymal progenitors in giant cell tumor of bone. Cancer Discov. 10, 1968–1987 (2020).
Street, K. et al. Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genomics 19, 477 (2018).
Angerer, P. et al. destiny: diffusion maps for large-scale single-cell data in R. Bioinformatics 32, 1241–1243 (2016).
Aran, D. et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat. Immunol. 20, 163–172 (2019).
Kowalczyk, M. S. et al. Single-cell RNA-seq reveals changes in cell cycle and differentiation programs upon aging of hematopoietic stem cells. Genome Res. 25, 1860–1872 (2015).
Vanner, R. J. et al. Quiescent Sox2+ cells drive hierarchical growth and relapse in Sonic hedgehog subgroup medulloblastoma. Cancer Cell 26, 33–47 (2014).
Newman, A. M. et al. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 12, 453–457 (2015).
Van de Sande, B. et al. A scalable SCENIC workflow for single-cell gene regulatory network analysis. Nat. Protoc. 15, 2247–2276 (2020).
Nagy, C. et al. Single-nucleus transcriptomics of the prefrontal cortex in major depressive disorder implicates oligodendrocyte precursor cells and excitatory neurons. Nat. Neurosci. 23, 771–781 (2020).
Gulati, G. S. et al. Single-cell transcriptional diversity is a hallmark of developmental potential. Science 367, 405–411 (2020).
Van den Berge, K. et al. Trajectory-based differential expression analysis for single-cell sequencing data. Nat. Commun. 11, 1201 (2020).
Therneau, T. A Package for Survival Analysis in R. R package version 3.4-0, https://CRAN.R-project.org/package=survival (2022).
Wei, Y. et al. A TAF4-homology domain from the corepressor ETO is a docking platform for positive and negative regulators of transcription. Nat. Struct. Mol. Biol. 14, 653–661 (2007).
Szklarczyk, D. et al. The STRING database in 2021: customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 49, D605–D612 (2021).
Merico, D., Isserlin, R., Stueker, O., Emili, A. & Bader, G. D. Enrichment Map: a network-based method for gene-set enrichment visualization and interpretation. PLoS ONE 5, e13984 (2010).
Shannon, P. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Gu, Z., Eils, R. & Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847–2849 (2016).
Zhou, X. et al. Exploring genomic alteration in pediatric cancer using ProteinPaint. Nat. Genet. 48, 4–6 (2016).
Penas, C. et al. Time series modeling of cell cycle exit identifies Brd4 dependent regulation of cerebellar neurogenesis. Nat. Commun. 10, 3028 (2019).
Reimand, J., Kull, M., Peterson, H., Hansen, J. & Vilo, J. g:Profiler—a web-based toolset for functional profiling of gene lists from large-scale experiments. Nucleic Acids Res. 35, W193–W200 (2007).
Acknowledgements
M.D.T. is supported by the NIH (R01NS106155, R01CA159859 and R01CA255369), The Pediatric Brain Tumor Foundation, The Terry Fox Research Institute, The Canadian Institutes of Health Research, The Cure Search Foundation, the Matthew Larson Foundation (IronMatt), b.r.a.i.n.child, Meagan’s Walk, the SWIFTY Foundation, The Brain Tumor Charity, Genome Canada, Genome BC, Genome Quebec, the Ontario Research Fund, Worldwide Cancer Research, the V-Foundation for Cancer Research and the Ontario Institute for Cancer Research through funding provided by the Government of Ontario. M.D.T. is also supported by a Canadian Cancer Society Research Institute Impact grant, a Cancer Research UK Brain Tumor Award and by a Stand Up To Cancer (SU2C) St Baldrick’s Pediatric Dream Team Translational Research Grant (SU2C-AACR-DT1113) and SU2C Canada Cancer Stem Cell Dream Team Research Funding (SU2C-AACR-DT-19-15) provided by the Government of Canada through Genome Canada and the Canadian Institutes of Health Research, with supplementary support from the Ontario Institute for Cancer Research through funding provided by the Government of Ontario. Stand Up to Cancer is a programme of the Entertainment Industry Foundation administered by the American Association for Cancer Research. M.D.T. is also supported by the Garron Family Chair in Childhood Cancer Research at the Hospital for Sick Children and the University of Toronto. K.J.M. is supported by NIH-R01-NS080390, R01-NS095733 and R37 NS095733. T.E.W.-O. and B. Doble are supported by the Canadian Institutes of Health Research (CIHR) and the CancerCare Manitoba Foundation. H.S. is supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (21K21001) and National Cancer Center Research and Development Funds (2021-A-1). C.L.K is supported by CIHR (PJT-156086), salary awards from Fonds de Recherche du Québec-Santé (FRQS) and NSERC (RGPIN-2016-04911). P. Haldipur is supported by NIH-R21 NS117848, Brain and Behavior Young Investigator Award. We also thank the staff at Human Developmental Biology Resource London and Newcastle for their technical help and support; B. Pakuts for support with graphic design and illustrations; the Fernando Goldsztein Family for supporting this research; and L. Rorke for thoughtful discussions on cerebellar dysplasia.
Author information
Authors and Affiliations
Contributions
L.D.H., P. Haldipur and O. Saulnier designed, performed and analysed the majority of the experiments in this study. J.M., A.H.S. and J.G. contributed to in situ hybridization and cell count analyses. A.W.E. contributed to bioinformatics analysis and SCENIC workflow. W.O. and D.S.S. contributed to cell line preparation for single-nucleus sequencing. W.O. and M.C.V. contributed to primary tissue isolation, single-cell preparation and sequencing. V.G. and L.C.-M. contributed to TurboID experiments and OTX2-KD experiments. A.L.M. contributed to GCP isolation and design and overexpression of the DN-CBFA2T3 construct. M.S. and T.P.-B. contributed to fusion CBFA2T2-TurboID construct design. M.L., R.A.S. and D. S. Scott contributed to CBFA2T2 mutation validation. S.B. contributed to western blot validations. H.F. contributed to SNP6 copy number analyses. T.N. and S.N. contributed to mutation analysis. A.B. contributed to pathway analysis. J.J.D.-M. contributed to CNV analysis of the scRNA-seq data. K.B. contributed to analysis and model design. P.S. and E.Y.W. contributed to RNA-seq fusion calling and analysis. O. Sirbu, S.A.K., P.B., A.V., J.J.Y.L., R.A., Xin Chen, Xiaodi Chen and K.L.M. contributed to experimental, statistical, bioinformatics analysis and/or interpretation. B. Luu, P. Bérubé, Y.C.W., A.S.M. and X.W. contributed to RNA-seq library preparation. S.M.P., N.J., S.-K.K., F.D., O.D., F.B., J.M.-P., W.A.G., J.L., P.D., M.F.-M., A.J., P.J.F., J.M.K., K.Z., S.D.B., C.G.E., A.A.N.R., C.G., J.M.O., M.G., P. Hauser, J.J.P., Y.S.R., C.d.T., J. Mora, K.K.W.L., H.-K.N., W.S.P., I.F.P., E.L.-A., G.Y.G., T.E.V.M., T.S., R.V., R.C.T., M.K.C., J.B.R., T.K., S.J., B. Lach, A.I., V.F., P.d.A., M.Z., G.C., S.R., D. S. Stearn, E.G.V.M., P.P., G.F., M.M., C.G.C., C.C.F., M.F.R., F. Boop, J.A.C., R.E.M., E.M.T., M.A., M.L.G., F.C., P.E. and M.P. provided patient tumour material and helped design the study. K.A.A., A.S.M., F.M.G.C., J.N.R., S.J.M.J., R.A.M., M.A.M., X.H., J.R., P.H.S., R.J.W.-R., W.A.W., T.J.P., L.G., C.L.K., L.D.S., N.J., D.M. and O.A. helped analyse data and provided expert advice. F.R. and E.S. provided late-gestation human samples. X.W. and C.D. provided reagents, equipment and expert advice. D.W.E. and J.A.G. provided expert neuropathology analysis and images. T.E.W.-O. and B.D. contributed to and provided expert advice on TurboID and OTX2-KD experiments. V.R. provided MRI images and provided expert advice on their interpretation. H.S., T.E.W.-O., K.J.M. and M.D.T. jointly supervised the project. L.D.H., P.H. and M.D.T. prepared the figures and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Mutations and copy number losses targeting the same genes are mutually exclusive in G3 and G4 MB.
a, b, c, Oncoprint summarizing gene mutations and copy number losses in their corresponding genomic loci (chromosome arm) for CHD (a), FANC (b) and ELP (c) driver families. Mutations are less frequent than copy number losses but tend to occur independently suggesting they are targets of the deletions. d, Mutual exclusivity of mutation and copy number loss events targeting genes in the CHD, FANC, and ELP families. e, Overlap between events targeting CHD, FANC, or ELP genes. Most G4 MB tumours are haploinsufficient for genes in at least two families through a single deletion event.
Extended Data Fig. 2 Clustering of G4 MB driver genes in the human genome predisposes humans to develop MB.
a, Cartoon of the Homo sapiens genome with the locations of known and newly identified G3 and G4 MB candidate driver genes demonstrating clustering of genes at locations known to be deleted in G3 and G4 MB. b, Frequency of whole chromosome arm loss is significantly correlated with the number of driver gene families—as detailed in (a)—contained on the arm. Significance was assessed by a two-sided linear regression model; grey shaded area denotes the 95% confidence interval. c, Mutual exclusivity of copy number losses of chromosome arms 17p, 16q, and 8q. Significance was assessed using the impurity test for mutual exclusivity, implemented in the R package DISCOVER41.
Extended Data Fig. 3 CBFA2T3 is a G4 MB tumour suppressor gene.
a, PRDM6 expression in CBFA2T2 mutant (red) and CBFA2T2 WT (grey) G3 and G4 MB samples demonstrates that enhancer hijacking mediated PRDM6 expression is largely limited to CBFA2T2 WT cases. b, Density of regions of chromosomal gain and loss along human chromosome 16q in G3 and G4 MB cases, demonstrating that deletions are biased towards the telomeric end of 16q, the location of known drivers, particularly CBFA2T3. c, CBFA2T3 expression differences between samples with and without CBFA2T3 deletions, split by subgroup. Statistical significance was assessed using Kruskal-Wallis rank-sum test (FDR < 0.05), *** p < 0.0005, G3, p = 2.88e−05; G4, p = 2.60e−09. G3, n = 112; G4, n = 206. CBFA2T3 is a copy-number responsive tumour suppressor gene in G4 MB. d, IGV analysis showing focal deleted region in two G4 MB samples MB-0364 and MB-0559. MB-0364, which is the minimal common deleted region (MCDR) on 16q in G3 and G4 MB, though does not quite achieve statistical significance in the GISTIC analysis. MB-0559 is the MCDR achieving statistical significance in GISTIC analysis. CBFA2T3 is identified with a red box. e, Cartoon illustrating the MCDR concept. f, Expression differences between copy neutral or hemizygously deleted G3 and G4 MB samples for genes within the MB-0364 MCDR on chr16q24.3. Statistical significance was assessed using two-sided Mann-Whitney U tests with FDR adjustment, * p < 0.05, *** p < 0.0005. Deletion, n = 86; Neutral, n = 232. e, Expression differences between copy neutral or hemizygously deleted G3 and G4 MB samples for genes within the MB-0559 MCDR on chr16q24.3. Statistical significance was assessed using two-sided Mann-Whitney U tests with FDR adjustment, * p < 0.05, *** p < 0.0005. Deletion, n = 86; Neutral, n = 232. A full list of p values for genes presented in (f) and (g) can be found in Supplementary Data Table 1. h, CBFA2T2 (left) and CBFA2T3 (right) expression in SHH, G3, and G4 MB by bulk RNAseq. Statistical significance was assessed by Kruskal-Wallis rank-sum test (FDR < 0.05), * p < 0.05, *** p < 0.0005. For CBFA2T2: SHH-G3, p = 2.29e−47; SHH-G4, p = 4.42e−73; G3-G4, p = 0.035. For CBFA2T3: SHH-G3, p = 7.10e−42; SHH-G4, p = 1.13e−46; G3-G4, p = 0.61. G3, n = 219; G4, n = 326; SHH, n = 250. While CBFA2T2 and CBFA2T3 are recurrently targeted and have low expression in G3 and G4 MB, high expression of both genes and an absence of alterations are observed in SHH MB. CBFA2T2 and CBFA2T3 likely have different roles in SHH MB compared to G3 and G4 MB. For c, f, g, and h box plots show the median and interquartile range, and whiskers show the data range. Points outside this range are outliers and are plotted individually.
Extended Data Fig. 4 The CBFA polyprotein complex contains multiple known and novel G4 MB driver genes.
a, Western blot showing successful expression of the TurboID-CBFA2T2 fusion protein when the TurboID construct is fused to the N-terminal of CBFA2T2, but not to the C-terminal. b, Protein-protein interaction (PPI) network of novel CBFA2T2 interacting proteins. Each node represents a protein and edges between the proteins represent known or novel PPIs. Edges in red represent known interactions between CBFA2T2 interacting proteins, and edges in green represent known interactions with CBFA2T2 that were recapitulated in our TurboID screen. Proteins are grouped with dashed lines if they contain known interactions between each other. c, Significant CBFA2T2 prey proteins enriched in each indicated biological process. GLI2 is a SHH oncogene and has been recently shown to maintain GCP proliferation and identity, implicating the CBFA complex82. d, Enrichment map of biological processes (GO:BP) enriched in CBFA2T2 prey proteins by TurboID. Each node represents a significantly enriched pathway and edges represent shared genes between nodes. Nodes are grouped and labelled with a common biological theme. Significance was assessed using G:Profiler83 with FDR correction. e, Protein-protein interaction (PPI) network of CBFA2T2 TurboID proteins and G3/G4 MB driver genes (Fig. 1a) using STRING41. Edges between CBFA2T2 and diamond-shaped nodes are not drawn for simplicity. Connectivity significance was assessed by STRING, p < 0.1e−16.
Extended Data Fig. 5 Disruption to the CBFA complex explains most G4 MB tumours.
a, Per cent of G3 and G4 MB in our cohort (n = 545) explained by alterations in genes connected to CBFA2T2 with a known or novel PPI (one step in the network presented in Extended Data Fig. 4e). Significance assessed using the impurity test for mutual exclusivity implemented in the R package DISCOVER41. b, Ranked expression of HBEGF (left) and EREG (right) in G4 MB (n = 326). Points are coloured by the presence (red) or absence (grey) of known CBFA complex alterations. Samples with the highest expression of HBEGF and EREG typically do not have CBFA complex alterations, suggesting an alternate mechanism of CBFA complex inhibition. Data presented in a were not performed in replicates. c, Summary of disrupted pathways in G3 and G4 MB. Altered genes are grouped by pathway and labelled with alteration frequency. d, (Left) H&E–stained midsagittal section from 17 PCW human cerebellum. (Right) Fluorescence immunohistochemistry (IHC) showing KI67 and SOX2 expression in the human RL compartments. Data presented in d were not performed in replicates. RLVZ and RLSVZ are denoted by red and yellow asterisks, respectively. Scale bars: 100 µm.
Extended Data Fig. 6 LMX1A expression distinguishes the two downstream lineages of the RLSVZ.
a, (Top left) In situ hybridization (ISH) showing MKI67 expression. In-set highlights the developing cerebellum, and the RL is indicated by the black box. (Other images) Hematoxylin and eosin (H&E)–stained midsagittal sections of the developing human cerebellum. In each, the rhombic lip is indicated by the black box. Scale bars: 500 µm. b, GFAP expression in the developing human RL at 17 PCW. Scale bar: 100 µm. The RLVZ and RLSVZ are physically divided by a vascular plexus, as indicated with white asterisks. c, ISH showing spatially resolved RNA expression of HBEGF in the developing human cerebellum at 17 PCW. Scale bar: 50 µm. HBEGF foci are enriched along the RL vascular plexus. d, KI67 expression in the developing human RL at 19 PCW. Scale bar: 100 µm. e, H&E–stained midsagittal sections of the 9-month postnatal human cerebellum. Scale bar: 500 µm. The RL is only present during gestation and disappears around birth. f, g, ISH showing spatially resolved RNA expression of CBFA2T2 (f) and CBFA2T3 (g) in the developing human cerebellum at 14 PCW. Scale bars: 100 µm. h, i, ISH showing spatially resolved RNA expression of Cbfa2t2 (h) and Cbfa2t3 (i) in the developing mouse cerebellum at E15.5 (Left) and E16.5 (Right). Scale bars: 100 µm. We do not observe a similar expression pattern of either gene in the mouse RL as we do in the human RL, and note an enrichment of expression in the EGL, similar to humans. j, ISH showing spatially resolved RNA expression of LMX1A in the developing human cerebellum at 11, 14, and 17 PCW. LMX1A is highly expressed in both the RLVZ and RLSVZ, but LMX1A expression is only retained in UBCs migrating away from the RL and is completely absent in GCPs that migrate to the EGL. Data presented in d is a representative image from three independent experiments with similar results, data in remaining panels were not performed in replicates.
Extended Data Fig. 7 Characterization of single cells used in transcriptional mapping between MB and human cerebellum development.
a, Dot plot showing expression of characteristic marker genes across RL glutamatergic cell types in the developing human cerebellum13. b, UMAP embeddings coloured by pseudotime inferred from Slingshot65, where the direction of pseudotime is from dark to light colours, for the granule cell lineage (Left) and the UBC lineage (Right). c, Expression of CBFA2T2 (Left) and CBFA2T3 (Right) in each zone of the developing human RL by bulk RNAseq12. Statistical significance was assessed using a two-sided Mann-Whitney U test, * p < 0.05; CBFA2T2, p = 0.0078; CBFA2T3, p = 0.0056. n = 9 biological samples, per zone, acquired between 9 and 19 PCW. Box plots show the median and interquartile range, and whiskers show the data range. Points outside this range are outliers and are plotted individually. d, UMAP embedding of 63,296 single cells derived from G3 (n = 6), G4 (n = 11), and SHH (n = 3) MB scRNAseq samples. Clusters of transcriptionally similar cells are colored and labeled by tumour sample or annotated cell type for non-tumour cells. e, Copy number variations detected in single cells inferred using inferCNV63. (Top) Reference non-tumour cells are devoid of copy number variations. (Bottom) Tumour cell clusters were enriched for copy number variations characteristic of the sample subgroup. Cells containing CNVs were assigned as tumour cells for downstream analysis. f, UMAP embedding as in (d) coloured by the detection of copy number variations. g, Dot plot showing expression of characteristic marker genes of SHH, G3, G4 MB, and the non-tumour cell types identified. For a, g, colour indicates average expression and size of each dot indicates the per cent of cells in that cluster that express the genes.
Extended Data Fig. 8 G3 and G4 MB resemble specific components of the human RL, whose differentiation is stalled in time.
a, SingleR67 classification of tumour cells from G3 (n = 6), G4 (n = 11), and SHH (n = 3) MB scRNAseq samples, by comparison to the entire developing human cerebellum13. As expected, MB cells are most similar to glutamatergic cells. b, SingleR classification of tumour cells from (a) by comparison to glutamatergic cell types. c, Kaplan-Meier plot showing overall survival of G3 and G4 MB subtypes in the current dataset. Significance assessed using a log-rank test. Censored cases, +. d, Relative confidence of per cell classifications, calculated as the average similarity score per subgroup (b), minus median similarity scores from other subgroups per cell type. e, UMAP embedding of n = 545 G3 and G4 MB bulk RNAseq samples, coloured by presence of KBTBD4 mutations. G4ɣ which are impoverished for CBFA complex mutations, and display high OTX2 expression, are also enriched for KBTBD4 mutations. f, Expression of super enhancer (SE) genes in the developing human cerebellum snRNA-seq data. These gene promoters have been demonstrated to promote transcription of PRDM6 (SNCAIP) and GFI1B (DDX31/BARHL1 and PRRC2B) in G3 and G4 MB secondary to enhancer hijacking events11,28. Significance was assessed using a two-sided Wilcoxon Rank Sum test with FDR correction, *** p < 0.0005. SNCAIP, p = 3.39e−261; DDX31, p = 4.27e−71; BARHL1, p = 4.91e−40. n = 9,208 cells. g, Expression of DDX31, BARHL1, and PRRC2B across all cell types in the developing human cerebellum. DDX31 and BARHL1 exhibit correlated expression specific to the RLSVZ, while PRRC2B is non-specifically expressed. Significance was assessed using a two-sided Wilcoxon Rank Sum test with FDR correction, *** p < 0.0005. DDX31, p = 3.66e−113; BARHL1, p = 6.26e−191. n = 59,608 cells. h, Expression of G3 and G4 MB driver genes (from Fig. 1a) in the developing human cerebellum snRNA-seq data. i, j, k, Average expression of all G3 and G4 MB driver genes (i), CBFA complex genes (j), and gain of function (GOF) driver genes (k) in the developing human cerebellum snRNA-seq data. Significance was assessed using a two-sided Wilcoxon Rank Sum test, * p < 0.05, *** p < 0.0005. n = 9,208 cells. (i), p = 1.5e−13; (j) GCP, p = 0.0085; Early GN, p = 0.047; (k) RLSVZ, p = 1.9e−05; GCP, p = 3.2e−13. For i, j, and k, box plots show the median and interquartile range, and whiskers show the data range. Points outside this range are outliers and are plotted individually.
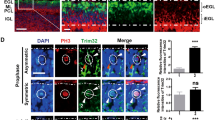
Extended Data Fig. 9 Human EOMES+ve RL cells are mitotically active UBC progenitors.
a, EOMES and PAX6 expression in the developing human RL at 17 (i) and 19 (ii) PCW. Scale bars: 100 µm. b, EOMES and KI67 expression in the developing human RL at 17 (i) and 19 (ii) PCW. Scale bars as in a. Proliferating EOMES+ve UBC progenitors are common across all developmental timepoints assessed. c, EOMES+ cells in the human RL zones. The RLSVZ contains significantly more EOMES+ cells that the RLVZ. Significance was assessed using an unpaired two-tailed t-test, *** p = 1.048e−18. n = 3 biological repeats, per N = 4 time points; error bars, SEM. d, EOMES and KI67 expression in the developing human RL at the late timepoint 30 PCW. Scale bar as in (a). Proliferating EOMES+ve UBC progenitors can be found across fetal development, though at reduced frequency at later time points as KI67 expression is reduced (Fig. 3c). e, Quantification of the number of EOMES+/KI67+ cells in the human RL across various developmental timepoints. All comparisons to 11 PCW were non-significant using two-tailed unpaired t-tests; 14PCW, p = 0.43; 17PCW, p = 0.65; 19PCW, p = 0.33. n = 3 biological repeats per timepoint; error bars, SEM. EOMES+/KI67+ UBC progenitors are a long-lived and dominant population of the RL, rather than a transient state preceding differentiation. f, Quantification of the number of Eomes+/Ki67+ cells in the mouse RL across various developmental timepoints. Significance was assessed using an unpaired two-tailed t-test, *** p = 0.00015. n = 3 biological repeats per timepoint; error bars, SEM. Eomes+/Ki67+ UBC progenitor cells are a rare population in the mouse RL. g, h, Eomes and Ki67 expression in the mouse RL at E15.5 (g) and E16.5 (h). The RL boundaries are indicated with white dashed lines. Scale bars: 50µm. Eomes+ UBCs are rarely Ki67+. Data presented in a, b, g, h are representative images from three independent experiments with similar results, data in d were not performed in replicates. i, Oncogenic divergence of RLSVZ progenitors from normal initiate G4 MB.
Extended Data Fig. 10 The location of G3 and G4 MB tumours coincides with OTX2 expression and supports an RL cell of origin.
a, Scaled OTX2, CBFA2T2, and CBFA2T3 expression by scRNAseq. b, Expression of OTX2 at 14 and 17 PCW by ISH in the developing human cerebellum. c, T1 enhanced or T2 mid-sagittal MRI images of G4 MB (n = 12) tumours at initial diagnosis. d, T1 enhanced or T2 mid-sagittal MRI images of G3 MB (n = 10) tumours at initial diagnosis. Both G3 and G4 MB tumours present exclusively in the OTX2+ inferior cerebellum. e, Axial T1 enhanced, T2 or FLAIR images of SHH MB (n = 3) at initial diagnosis. SHH tumours occur in the cerebellar hemispheres, consistent with an EGL cell of origin. f, Axial T1 enhanced, T2 or FLAIR images of WNT MB (n = 3) at initial diagnosis. Data presented in b were not performed in replicates.
Extended Data Fig. 11 OTX2 knockdown promotes G3 MB differentiation through intermediate upregulation of CBFA2T2 and CBFA2T3.
a, OTX2 ChIP-seq34 peaks are enriched at CBFA2T2 gene locus, but not CBFA2T3. b, OTX2 protein expression is reduced following OTX2-KD. Samples were used for bulk RNA sequencing. Beta actin used as a loading control. c, OTX2 protein expression is reduced following OTX2-KD. Samples were used for single-nucleus RNA sequencing. Beta actin used as a loading control. d, Representative images of primary tumourspheres in OTX2-KD and scramble conditions for both HDMB03 and MB3W1 cultures. Scale bar: 300 µm. e, f, Unbiased clustering of single nuclei following OTX2-KD in HDMB03 and MB3W1 G3 MB cells lines (c). g, Average expression of gene signatures derived from bulk RNAseq on OTX2-KD from HDMB03 and MB3W1 (b). Cells that are more orange than green indicate cells with higher expression of genes characteristic of the unchanged G3 MB cell lines, and vice-versa. Orange cells are likely cells where OTX2-KD was inefficient. h, Differentiation score as determined by CytoTRACE73. Less differentiated cells are indicated in red and more differentiated cells are indicated in blue. The results support a model where cluster 6 in HDMB03 and cluster 3 in MB3W1 represent inefficient OTX2-KD cells that retain the most similarity to WT tumour cells. i, RBFOX3 (NeuN) protein expression is increased following OTX2-KD in both HDMB03 and MB3W1, validating GN differentiation following OTX2-KD. j, Expression of genes significantly correlated with granule neuron differentiation along pseudotime in normal human RL development. (Top) Density of cell along pseudotime in the granule neuron lineage (Extended Data Fig. 6b, left). (Bottom) Binned gene expression of markers derived from the developing human cerebellum snRNA-seq dataset (Fig. 4a). The stepwise expression of granule neuron genes observed when OTX2 is knocked down in G3 MB (Fig. 5i) strikingly mirrors that of normal granule neuron differentiation, suggesting that G3 and G4 MB arise from failed normal differentiation rather than alternate hypotheses, such as trans- or de-differentiation. k, CBFA2T2 and CBFA2T3 expression in HDMB03 (Left) and MB3W1 (Right). CBFA2T2 expression is strongly upregulated in cells where the CytoTRACE score drops below 0.8, and CBFA2T3 follows. The results suggest CBFA2T2 and then CBFA2T3 are upregulated early in response to efficient OTX2-KD. l, CBFA2T2 expression change in response to CBFA2T2 overexpression (CBFA2T2-OE) in HDMB03 by qPCR. m, CBFA2T2 protein expression is increased following CBFA2T2-OE. β-actin used as a loading control. n, Representative images of primary tumourspheres in CBFA2T2-OE GFP and Control RFP conditions. Scale bar: 600 µm. o, p, Live cell number (o) and viability (p) in response to CBFA2T2-OE. Live cell number is significantly reduced in response to CBFA2T2-OE, while viability is unchanged. Data are normalized to their respective controls and presented showing points from n = 3 technical replicates per N = 8 or N = 5 biological replicates, for live cell number and viability, respectively. Error bars indicate SEM. Significance assessed using a two-tailed paired t-test on biological replicates, ** p = 0.0047. q, OTX2 restrains differentiation of RL progenitors through CBFA complex inhibition. Data presented in d, n are representative images from 4 and 8 independent experiments, respectively, with similar results, data in b, c, i, m were not performed in replicates. For gel source data, see Supplementary Fig. 1
Supplementary information
Supplementary Information
This file includes Supplementary Fig. 1 (the uncropped blots) and full descriptions for Supplementary Tables 1–7.
Supplementary Table 1
Significance values for 16q deleted genes.
Supplementary Table 2
Human MB sample metadata.
Supplementary Table 3
CBFA2T2 TurboID proteins.
Supplementary Table 4
Genes differentiating the subtypes of G3 and G4 MB.
Supplementary Table 5
Mutation calls in G3 and G4 MB.
Supplementary Table 6
scRNA-seq and snRNA-seq alignment metrics.
Supplementary Table 7
Top differentially expressed gene markers of cell types in human glutamatergic cell development.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hendrikse, L.D., Haldipur, P., Saulnier, O. et al. Failure of human rhombic lip differentiation underlies medulloblastoma formation. Nature 609, 1021–1028 (2022). https://doi.org/10.1038/s41586-022-05215-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05215-w
This article is cited by
-
Heterogeneity and tumoral origin of medulloblastoma in the single-cell era
Oncogene (2024)
-
Revisiting the development of cerebellar inhibitory interneurons in the light of single-cell genetic analyses
Histochemistry and Cell Biology (2024)
-
Evolution of neurosurgical advances and nuances in medulloblastoma therapy
Child's Nervous System (2024)
-
MYC overexpression and SMARCA4 loss cooperate to drive medulloblastoma formation in mice
Acta Neuropathologica Communications (2023)
-
Common molecular features of H3K27M DMGs and PFA ependymomas map to hindbrain developmental pathways
Acta Neuropathologica Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.