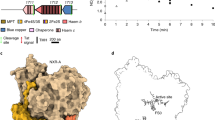
Abstract
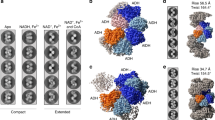
Filamentous enzymes have been found in all domains of life, but the advantage of filamentation is often elusive1. Some anaerobic, autotrophic bacteria have an unusual filamentous enzyme for CO2 fixation—hydrogen-dependent CO2 reductase (HDCR)2,3—which directly converts H2 and CO2 into formic acid. HDCR reduces CO2 with a higher activity than any other known biological or chemical catalyst4,5, and it has therefore gained considerable interest in two areas of global relevance: hydrogen storage and combating climate change by capturing atmospheric CO2. However, the mechanistic basis of the high catalytic turnover rate of HDCR has remained unknown. Here we use cryo-electron microscopy to reveal the structure of a short HDCR filament from the acetogenic bacterium Thermoanaerobacter kivui. The minimum repeating unit is a hexamer that consists of a formate dehydrogenase (FdhF) and two hydrogenases (HydA2) bound around a central core of hydrogenase Fe-S subunits, one HycB3 and two HycB4. These small bacterial polyferredoxin-like proteins oligomerize through their C-terminal helices to form the backbone of the filament. By combining structure-directed mutagenesis with enzymatic analysis, we show that filamentation and rapid electron transfer through the filament enhance the activity of HDCR. To investigate the structure of HDCR in situ, we imaged T. kivui cells with cryo-electron tomography and found that HDCR filaments bundle into large ring-shaped superstructures attached to the plasma membrane. This supramolecular organization may further enhance the stability and connectivity of HDCR to form a specialized metabolic subcompartment within the cell.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Cryo-EM maps, as well as cryo-ET subtomogram averages and cellular tomograms are available in the Electron Microscopy Data Bank (EMDB) with the accession codes EMD-14169 (cryo-EM map), EMD- 15053 (subtomogram average of HDCR), EMD-15054 (subtomogram average of T. kivui ribosomes), EMD-15055 (Fig. 5b tomogram) and EMD-15056 (Fig. 5a tomogram). Raw electron tomography data are available in the Electron Microscopy Public Image Archive (EMPIAR-11058). The atomic model of HDCR is available in the PDB (7QV7). Structural and sequence data used for comparison with HDCR subunits are available in the PDB (3C8Y, iron hydrogenase from Clostridium pasteurianum; 1H0H, W-containing formate dehydrogenase from D. gigas). Source data are provided with this paper.
References
Park, C. K. & Horton, N. C. Structures, functions, and mechanisms of filament forming enzymes: a renaissance of enzyme filamentation. Biophys. Rev. 11, 927–994 (2019).
Schuchmann, K. & Müller, V. Direct and reversible hydrogenation of CO2 to formate by a bacterial carbon dioxide reductase. Science 342, 1382–1385 (2013).
Schwarz, F. M., Schuchmann, K. & Müller, V. Hydrogenation of CO2 at ambient pressure catalyzed by a highly active thermostable biocatalyst. Biotechnol. Biofuels 11, 237 (2018).
Sordakis, K. et al. Homogeneous catalysis for sustainable hydrogen storage in formic acid and alcohols. Chem. Rev. 118, 372–433 (2018).
Müller, V. New horizons in acetogenic conversion of one-carbon substrates and biological hydrogen storage. Trends Biotechnol. 37, 1344–1354 (2019).
Scheffers, B. R. et al. The broad footprint of climate change from genes to biomes to people. Science 354, aaf7671 (2016).
Pecl, G. T. et al. Biodiversity redistribution under climate change: impacts on ecosystems and human well-being. Science 355, eaai9214 (2017).
DeWeerdt, S. Sea change. Nature 550, S54–S58 (2017).
Masson-Delmotte, V. et al. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC, 2021).
Ripple, W. J. et al. World scientists' warning to humanity: a second notice. Bioscience 67, 1026–1028 (2017).
Rand, D. A. J. & Dell, R. M. Hydrogen Energy: Challenges and Prospects (Royal Society of Chemistry, 2007).
Chu, S. & Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 488, 294–303 (2012).
Fukuzumi, S. Bioinspired energy conversion systems for hydrogen production and storage. Eur. J. Inorg. Chem. 2008, 1351–1362 (2008).
Joo, F. Breakthroughs in hydrogen storage—formic acid as a sustainable storage material for hydrogen. ChemSusChem 1, 805–808 (2008).
Loges, B., Boddien, A., Gärtner, F., Junge, H. & Beller, M. Catalytic generation of hydrogen from formic acid and its derivatives: useful hydrogen storage materials. Top. Catal. 53, 902–914 (2010).
Mellmann, D., Sponholz, P., Junge, H. & Beller, M. Formic acid as a hydrogen storage material—development of homogeneous catalysts for selective hydrogen release. Chem. Soc. Rev. 45, 3954–3988 (2016).
Eppinger, J. & Huang, K.-W. Formic acid as a hydrogen energy carrier. ACS Energy Lett. 2, 188–195 (2016).
Enthaler, S., von Langermann, J. & Schmidt, T. Carbon dioxide and formic acid—the couple for environmental-friendly hydrogen storage? Energy Environ. Sci. 3, 1207–1217 (2010).
Agarwal, A. S., Zhai, Y., Hill, D. & Sridhar, N. The electrochemical reduction of carbon dioxide to formate/formic acid: engineering and economic feasibility. ChemSusChem 4, 1301–1310 (2011).
Pereira, I. A. An enzymatic route to H2 storage. Science 342, 1329–1330 (2013).
Preuster, P., Papp, C. & Wasserscheid, P. Liquid organic hydrogen carriers (LOHCs): Toward a hydrogen-free hydrogen economy. Acc. Chem. Res. 50, 74–85 (2017).
Li, H. et al. Integrated electromicrobial conversion of CO2 to higher alcohols. Science 335, 1596 (2012).
Yishai, O., Lindner, S. N., Gonzalez de la Cruz, J., Tenenboim, H. & Bar-Even, A. The formate bio-economy. Curr. Opin. Chem. Biol. 35, 1–9 (2016).
Pinske, C. & Sargent, F. Exploring the directionality of Escherichia coli formate hydrogenlyase: a membrane-bound enzyme capable of fixing carbon dioxide to organic acid. MicrobiologyOpen 5, 721–737 (2016).
Wang, W. H., Himeda, Y., Muckerman, J. T., Manbeck, G. F. & Fujita, E. CO2 hydrogenation to formate and methanol as an alternative to photo- and electrochemical CO2 reduction. Chem. Rev. 115, 12936–12973 (2015).
Matubayasi, N. & Nakahara, M. Hydrothermal reactions of formaldehyde and formic acid: free-energy analysis of equilibrium. J. Chem. Phys. 122, 074509 (2005).
Kottenhahn, P., Schuchmann, K. & Müller, V. Efficient whole cell biocatalyst for formate-based hydrogen production. Biotechnol. Biofuels 11, 93 (2018).
Schwarz, F. M. & Müller, V. Whole-cell biocatalysis for hydrogen storage and syngas conversion to formate using a thermophilic acetogen. Biotechnol. Biofuels 13, 32 (2020).
Schuchmann, K., Vonck, J. & Müller, V. A bacterial hydrogen-dependent CO2 reductase forms filamentous structures. FEBS J. 283, 1311–1322 (2016).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Peters, J. W., Lanzilotta, W. N., Lemon, B. J. & Seefeldt, L. C. X-ray crystal structure of the Fe-only hydrogenase (Cpl) from Clostridium pasteurianum to 1.8 angstrom resolution. Science 282, 1853–1858 (1998).
Maia, L. B., Moura, I. & Moura, J. J. G. Molybdenum and tungsten-containing formate dehydrogenases: aiming to inspire a catalyst for carbon dioxide utilization. Inorganica Chim. Acta 455, 350–363 (2017).
Dong, G. & Ryde, U. Reaction mechanism of formate dehydrogenase studied by computational methods. J. Biol. Inorg. Chem. 23, 1243–1254 (2018).
Niks, D. & Hille, R. Molybdenum- and tungsten-containing formate dehydrogenases and formylmethanofuran dehydrogenases: structure, mechanism, and cofactor insertion. Protein Sci. 28, 111–122 (2019).
Maia, L. B., Moura, I. & Moura, J. J. G. in Enzymes for Solving Humankind's Problems: Natural and Artificial Systems in Health, Agriculture, Environment and Energy (eds Moura, J. J. G., Moura, I. & Maia, L. B.) 29–81 (Springer, 2021).
Raaijmakers, H. et al. Gene sequence and the 1.8 Å crystal structure of the tungsten-containing formate dehydrogenase from Desulfovibrio gigas. Structure 10, 1261–1272 (2002).
Page, C. C., Moser, C. C., Chen, X. & Dutton, P. L. Natural engineering principles of electron tunnelling in biological oxidation–reduction. Nature 402, 47–52 (1999).
Basen, M., Geiger, I., Henke, L. & Müller, V. A genetic system for the thermophilic acetogenic bacterium Thermoanaerobacter kivui. Appl. Environ. Microbiol. 84, e02210–e02217 (2018).
Jain, S., Dietrich, H. M., Müller, V. & Basen, M. Formate is required for growth of the thermophilic acetogenic bacterium Thermoanaerobacter kivui lacking hydrogen-dependent carbon dioxide reductase (HDCR). Front. Microbiol. 11, 59 (2020).
Esteve-Núñez, A., Sosnik, J., Visconti, P. & Lovley, D. R. Fluorescent properties of c-type cytochromes reveal their potential role as an extracytoplasmic electron sink in Geobacter sulfurreducens. Environ. Microbiol. 10, 497–505 (2008).
Bewley, K. D., Ellis, K. E., Firer-Sherwood, M. A. & Elliott, S. J. Multi-heme proteins: Nature's electronic multi-purpose tool. Biochim. Biophys. Acta 1827, 938–948 (2013).
Sturm, G. et al. A dynamic periplasmic electron transfer network enables respiratory flexibility beyond a thermodynamic regulatory regime. ISME J. 9, 1802–1811 (2015).
Schaffer, M. et al. Optimized cryo-focused ion beam sample preparation aimed at in situ structural studies of membrane proteins. J. Struct. Biol. 197, 73–82 (2017).
Asano, S., Engel, B. D. & Baumeister, W. In situ cryo-electron tomography: a post-reductionist approach to structural biology. J. Mol. Biol. 428, 332–343 (2016).
Bäuerlein, F. J. B. & Baumeister, W. Towards visual proteomics at high resolution. J. Mol. Biol. 433, 167187 (2021).
Schuchmann, K. & Müller, V. Autotrophy at the thermodynamic limit of life: a model for energy conservation in acetogenic bacteria. Nat. Rev. Microbiol. 12, 809–821 (2014).
Schoelmerich, M. C. & Müller, V. Energy conservation by a hydrogenase-dependent chemiosmotic mechanism in an ancient metabolic pathway. Proc. Natl Acad. Sci. USA 116, 6329–6334 (2019).
Schwarz, F. M., Moon, J., Oswald, F. & Müller, V. Biological hydrogen storage and release through multiple cycles of bi-directional hydrogenation of CO2 to formic acid in a single process unit. Joule 6, 1304–1319 (2022).
Debabov, V. G. Acetogens: biochemistry, bioenergetics, genetics, and biotechnological potential. Microbiology 90, 273–297 (2021).
Roger, M., Reed, T. C. P. & Sargent, F. Harnessing Escherichia coli for bio-based production of formate under pressurized H2 and CO2 gases. Appl. Environ. Microbiol. 87, e00299–00221 (2021).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Biyani, N. et al. Focus: the interface between data collection and data processing in cryo-EM. J. Struct. Biol. 198, 124–133 (2017).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Zhang, K. Gctf: real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Punjani, A., Zhang, H. & Fleet, D. J. Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nat. Methods 17, 1214–1221 (2020).
Tan, Y. Z. et al. Addressing preferred specimen orientation in single-particle cryo-EM through tilting. Nat. Methods 14, 793–796 (2017).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D 74, 531–544 (2018).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Moriarty, N. W., Grosse-Kunstleve, R. W. & Adams, P. D. electronic Ligand Builder and Optimization Workbench (eLBOW): a tool for ligand coordinate and restraint generation. Acta Crystallogr. D 65, 1074–1080 (2009).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010).
Delano, W. L. The PyMOL Molecular Graphics System (Schrödinger, 2002).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Shaw, A. J., Hogsett, D. A. & Lynd, L. R. Natural competence in Thermoanaerobacter and Thermoanaerobacterium species. Appl. Environ. Microbiol. 76, 4713–4719 (2010).
Benner, P. Proteinproduktion im Thermophilen, Acetogenen Bakterium Thermoanaerobacter kivui. BSc thesis, Goethe Univ. (2016).
Gibson, D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009).
Bradford, M. M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of proteine-dye binding. Anal. Biochem. 72, 248–254 (1976).
Wolff, G. et al. Mind the gap: micro-expansion joints drastically decrease the bending of FIB-milled cryo-lamellae. J. Struct. Biol. 208, 107389 (2019).
Hagen, W. J. H., Wan, W. & Briggs, J. A. G. Implementation of a cryo-electron tomography tilt-scheme optimized for high resolution subtomogram averaging. J. Struct. Biol. 197, 191–198 (2017).
Wan, W. williamnwan/TOMOMAN: TOMOMAN v.08042020 https://doi.org/10.5281/zenodo.4110737 (Zenodo, 2020).
Grant, T. & Grigorieff, N. Measuring the optimal exposure for single particle cryo-EM using a 2.6 Å reconstruction of rotavirus VP6. eLife 4, e06980 (2015).
Mastronarde, D. N. & Held, S. R. Automated tilt series alignment and tomographic reconstruction in IMOD. J. Struct. Biol. 197, 102–113 (2017).
Kremer, J. R., Mastronarde, D. N. & McIntosh, J. R. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 116, 71–76 (1996).
Buchholz, T., Jordan, M., Pigino, G. & Jug, F. Cryo-CARE: Content-aware image restoration for cryo-transmission electron microscopy data. In 2019 IEEE 16th International Symposium on Biomedical Imaging (ISBI 2019) 502–506 (IEEE, 2019).
Martinez-Sanchez, A., Garcia, I., Asano, S., Lucic, V. & Fernandez, J. J. Robust membrane detection based on tensor voting for electron tomography. J. Struct. Biol. 186, 49–61 (2014).
Wan, W. williamnwan/STOPGAP: STOPGAP v.0.7.1 https://doi.org/10.5281/zenodo.3973664 (Zenodo, 2020).
Turoňová, B., Schur, F. K. M., Wan, W. & Briggs, J. A. G. Efficient 3D-CTF correction for cryo-electron tomography using NovaCTF improves subtomogram averaging resolution to 3.4 Å. J. Struct. Biol. 199, 187–195 (2017).
Pintilie, G. D., Zhang, J., Goddard, T. D., Chiu, W. & Gossard, D. C. Quantitative analysis of cryo-EM density map segmentation by watershed and scale-space filtering, and fitting of structures by alignment to regions. J. Struct. Biol. 170, 427–438 (2010).
Harauz, G. & van Heel, M. Exact filters for general geometry three dimensional reconstruction. Optik 73, 146–156 (1986).
Rosenthal, P. B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 333, 721–745 (2003).
Grant, T., Rohou, A. & Grigorieff, N. cisTEM, user-friendly software for single-particle image processing. eLife 7, e35383 (2018).
Qu, K. et al. Structure and architecture of immature and mature murine leukemia virus capsids. Proc. Natl Acad. Sci. USA 115, E11751–E11760 (2018).
Acknowledgements
We thank D. Bollschweiler and T. Schäfer at the MPIB cryo-EM facility for single-particle cryo-EM data acquisition, J. Zarzycki for help with model building, M. Demulder for help with FIB milling and H. van den Hoek, F. Beck, P. Erdmann, S. Khavnekar and W. Wan for scripts and advice with cryo-ET analysis. We are grateful to E. Conti, J. Plitzko and W. Baumeister for access to state-of-the-art FIB and transmission electron microscopy instrumentation; to P. Benner and M. Basen for their gift of pPB5 and for discussions; and to L. Ribaric for help in preparing pLR2, pLR2c, pLR3b and pLR4. Calculations were performed at the Max Planck Institute for Biochemistry computing cluster in Martinsried, Germany, and at the sciCORE (http://scicore.unibas.ch/) scientific computing center at the University of Basel. J.M.S. acknowledges the DFG for early career support by an Emmy Noether grant (SCHU 3364/1-1). Work from the V.M. laboratory was supported by the European Research Council (Acetogens, grant agreement no. 741791). Work from the B.D.E. laboratory was supported by a DFG grant (EN 1194/1–1, part of FOR 2092), Helmholtz Munich and the University of Basel. H.M.D. was funded by a fellowship from Deutsche Bundesstiftung Umwelt (DBU) (PhD. grant no. 20016/446). R.D.R. acknowledges funding from the Alexander von Humboldt Foundation and a non-stipendiary fellowship from EMBO.
Author information
Authors and Affiliations
Contributions
H.M.D., B.D.E., J.M.S. and V.M. designed and coordinated the experiments. H.M.D., R.T. and F.M.S. expressed and purified the proteins. H.M.D. and R.T. carried out enzymatic assays. S.K.S. and J.M.S. collected and processed cryo-EM data. A.K., S.K.S. and J.M.S. built and refined models. H.M.D., R.D.R., A.K., J.M.S. and V.M. analysed and interpreted the functional and structural data. W.W. and J.W. performed FIB milling and cryo-ET data acquisition. R.D.R. and J.W. processed and analysed the cryo-ET data. A.K. and J.W. performed the negative-stain imaging. J.M.S., B.D.E. and V.M. wrote the manuscript together with all of the other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Alexey Amunts and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Cryo-EM data collection and analysis.
a, A representative cryo-EM micrograph (n = 33,853) collected on a FEI Titan Krios microscope (scale bar: 50 nm), operated at 300 kV and equipped with a K3 camera. b, Reference-free 2D class averages revealing the short HDCR filament in multiple orientations. c, Overview of the cryo-EM data-processing scheme. d, Angular distribution of the particles used for the final round of refinement. e, Plot showing the global resolution and sphericity of the final HDCR reconstruction, calculated using the “Remote 3DFSC Processing Server” web interface58. A sphericity of 0.939 indicates an isotropic particle orientation. f, Local resolution as calculated by CryoSPARC mapped on the refined density (left: bottom and side view, right: cut-open view of central section).
Extended Data Fig. 2 Filament bundling of purified HDCR used for cryo-EM and negative staining.
a–d, Longer HDCR filaments were occasionally observed in cryo-EM micrographs of the purified HDCR preparation. These filaments generally grouped together as bundles with varying filament length, impeding structural analysis. Representative images from 33,853 micrographs collected. Micrograph recording was performed as described in Extended Data Fig. 1. Scale bar: 50 nm. e–f, Representative negative-stain images of HDCR_His from F2 of Fig. 3f (n = 8), showing large filament bundles. Scale bars: 100 nm.
Extended Data Fig. 3 Model quality.
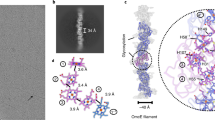
a, Structural models of the enzymatic active subunits in their electron density. FdhF domain IV is flexible (see Extended Data Fig. 5). The same colours are used as in Fig. 1. b, Representative regions of the HDCR complex and surrounding electron density maps are shown. Subunits and residue numbers are specified. Snapshots are shown for the density of both folded and cofactor binding regions.
Extended Data Fig. 4 Structural conservation of HydA2.
a, Structural model of HydA2. b, Superposition of HydA2 (blue) with the closest homolog [FeFe]-hydrogenase from Clostridium pasteurianum and zoom-in of the active site. c, Fit of the H-cluster (PDB: 3C8Y) in the electron density. d, Sequence alignment of HydA2 with the [FeFe]-hydrogenase CpI from Clostridium pasteurianum. Conserved residues are highlighted with colour, with darker shades of blue indicating high conservation. This alignment shows high conservation of the cap domain. Functional and cofactor-coordinating residues are marked according to the legend on the right side, revealing a full conservation of H-cluster coordination.
Extended Data Fig. 5 Structural conservation of FdhF.
a, Structural model of domains I-III of FdhF as built from the cryo-EM density. Close-up of the [4Fe4S]-cluster fitted into its map (mesh), demonstrating map quality. b, Superposition of FdhF (green) with the tungsten-containing formate dehydrogenase from D. gigas (pink, PDB: 1H0H). Close-up of the tungsten and pterin guanine dinucleotide binding site reveals high structural conservation. Fit of the W-bisPGD cofactors (1H0H) in the electron density. c, Composite model of FdhF: domains I-III were built from the cryo-EM density (as in panel a), and domain IV as well as the W-bisPGD cofactors were derived from homology. d, Sequence alignment of FdhF with the tungsten-containing formate dehydrogenase from D. gigas. Conserved residues are highlighted, with darker shades of blue indicating high conservation. This alignment shows that all domains are highly conserved. Functional and cofactor-coordinating residues are marked according to the legend on the right side, revealing conservation of W-bisPGD cofactor coordination. For more details on conserved W-bisPGD coordinating amino acids, see also Supplementary Table 2.
Extended Data Fig. 6 HDCR_His complements the native HDCR enzyme activity.
a, Purified HDCR (10 µg) from wild-type T. kivui (HDCR native) and from the overproduction strain HDCR_His have identical protein subunits. b, Isolated native HDCR and the HDCR_His tested for H2 evolution from formate and formate production from H2 + CO2. Data for “HDCR native” are reproduced from a previous study3. Hydrogen production from formate (150 mM) catalysed by 10 µg isolated HDCR_His. Formate production as described before, but H2 + CO2 (80:20 [v:v], 1.1 x 105 Pa) was used as a substrate. c, Hydrogen production from formate (150 mM) catalysed by 0.3 mg of cytoplasmic fractions of WT (HDCR native) and HDCR_His T. kivui strains. All data points are mean ± s.e.m., taken from 3 biologically independent replicates, each with 3 technical replicates. Statistical analysis was performed using one-way analysis of variance (ANOVA) with comparative Tukey post-hoc test (significance level ***p = 0.001).
Extended Data Fig. 7 Catalytic properties of HDCR variants.
a-d, Characterization of the pH- and temperature-dependence of HDCR native (squares) and HDCR_His (circles). a and c, Methylviologen-dependent hydrogenase activity with H2 or b and d, formate dehydrogenase activity with formate as electron donor. Data for HDCR native are reproduced from a previous study3. 0.03 µg (H2:MV-oxidoreductase activity) or 3 µg (formate:MV-oxidoreductase activity) of HDCR_His were incubated in reaction buffer at 64 °C. 10 mM methylviologen was used as an electron acceptor, and reduction of methylviologen was monitored at 604 nm. MV, methylviologen. e-f, Functionality of catalytical subunits in HDCR variants. e, Methylviologen-dependent hydrogenase activity with H2 or f, formate dehydrogenase activity with formate as an electron donor. 3 µg (H2:MV-oxidoreductase activity) or 30 µg (formate:MV-oxidoreductase activity) of cytoplasmic fractions containing HDCR variants were incubated in reaction buffer at 64 °C. 10 mM methylviologen was used as an electron acceptor, and reduction of methylviologen was monitored at 604 nm. 100 % corresponds to the activity of the complete HDCR_His complex (H2:MV-oxidoreductase activity 301 µmol min−1 mg−1; formate:MV-oxidoreductase activity 40 µmol min−1 mg−1). MV, methylviologen. g) Hydrogen production from formate of selected HDCR variants. h) Formate production from H2 + CO2 of selected HDCR variants. HDCR_His was defined as 100 % relative enzyme activity (hydrogen evolution from formate, 83 µmol min−1 mg−1; formate production from H2 + CO2, 25 µmol min−1 mg−1). All data points are mean ± s.e.m., taken from 3 (a–g) or 1 (h) biologically independent replicates, each with 3 (e,f,h) or 2 (a,b,c,d,g) technical replicates. Statistical analysis was performed using one-way analysis of variance (ANOVA) with comparative Tukey post-hoc test (significance level ***p = 0.001). For further methods details, see the Supplementary Information.
Extended Data Fig. 8 Cryo-ET of wild-type and Δhdcr mutant T. kivui cells confirms the identity of HDCR.
a–f, Slices through cryo-tomograms of wild-type (WT) T. kivui cells containing HDCR filament bundles (yellow arrowheads). HDCR filaments were observed in 22 of n = 34 WT tomograms. g–l, Slices through cryo-tomograms of mutant T. kivui cells in which the genes coding for HDCR proteins were deleted (Δhdcr). No filaments were observed in n = 34 Δhdcr tomograms. Slice thickness: 7 nm.
Extended Data Fig. 9 Overview of HDCR subtomogram averaging, and helical pitch comparison between in vitro and in situ structures.
a, Processing flowchart used for HDCR subtomogram averaging in situ. For additional details, see Methods. b, Fourier shell correlation (FSC) curves from the final subtomogram average (displayed in Fig. 5g). c, comparison of observed helical pitch in vitro (98.5 nm with rise: 68.4 Å, twist: 25°) and in situ (289.5 nm with rise: 67.8 Å, twist: 8.43°).
Supplementary information
Supplementary Information
Supplementary file containing additional information about HDCR subunit interaction, further method details, and the impact of filamentation for HDCR enzymes. Supplementary Tables 1–5 contain detailed information about cofactor coordination in HDCR, as well as primers, plasmids and strains used in this study. Supplementary Figure 1 includes uncropped polyacrylamide gels. The Supplementary Information also includes legends for Supplementary Videos and Supplementary References.
Rights and permissions
About this article
Cite this article
Dietrich, H.M., Righetto, R.D., Kumar, A. et al. Membrane-anchored HDCR nanowires drive hydrogen-powered CO2 fixation. Nature 607, 823–830 (2022). https://doi.org/10.1038/s41586-022-04971-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-04971-z
This article is cited by
-
Self Fourier shell correlation: properties and application to cryo-ET
Communications Biology (2024)
-
An allosteric redox switch involved in oxygen protection in a CO2 reductase
Nature Chemical Biology (2024)
-
Reprogramming the metabolism of an acetogenic bacterium to homoformatogenesis
The ISME Journal (2023)
-
Serial Lift-Out: sampling the molecular anatomy of whole organisms
Nature Methods (2023)
-
Strukturelle und mechanistische Grundlagen der Acetogenese
BIOspektrum (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.