Abstract
Time-restricted feeding (TRF) has recently gained interest as a potential anti-ageing treatment for organisms from Drosophila to humans1,2,3,4,5. TRF restricts food intake to specific hours of the day. Because TRF controls the timing of feeding, rather than nutrient or caloric content, TRF has been hypothesized to depend on circadian-regulated functions; the underlying molecular mechanisms of its effects remain unclear. Here, to exploit the genetic tools and well-characterized ageing markers of Drosophila, we developed an intermittent TRF (iTRF) dietary regimen that robustly extended fly lifespan and delayed the onset of ageing markers in the muscles and gut. We found that iTRF enhanced circadian-regulated transcription and that iTRF-mediated lifespan extension required both circadian regulation and autophagy, a conserved longevity pathway. Night-specific induction of autophagy was both necessary and sufficient to extend lifespan on an ad libitum diet and also prevented further iTRF-mediated lifespan extension. By contrast, day-specific induction of autophagy did not extend lifespan. Thus, these results identify circadian-regulated autophagy as a critical contributor to iTRF-mediated health benefits in Drosophila. Because both circadian regulation and autophagy are highly conserved processes in human ageing, this work highlights the possibility that behavioural or pharmaceutical interventions that stimulate circadian-regulated autophagy might provide people with similar health benefits, such as delayed ageing and lifespan extension.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available, including replicate experiments, and will be made available on reasonable request from the corresponding author.
References
Longo, V. D. & Panda, S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 23, 1048–1059 (2016).
Villanueva, J. E. et al. Time-restricted feeding restores muscle function in Drosophila models of obesity and circadian-rhythm disruption. Nat. Commun. 10, 2700 (2019).
Chaix, A., Zarrinpar, A., Miu, P. & Panda, S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 20, 991–1005 (2014).
Gill, S., Le, H. D., Melkani, G. C. & Panda, S. Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science 347, 1265–1269 (2015).
de Cabo, R. & Mattson, M. P. Effects of intermittent fasting on health, aging, and disease. N. Engl. J. Med. 381, 2541–2551 (2019).
Catterson, J. H. et al. Short-term, intermittent fasting induces long-lasting gut health and TOR-independent lifespan extension. Curr. Biol. 28, 1714-1724.e4 (2018).
Manoogian, E. N. C. & Panda, S. Circadian rhythms, time-restricted feeding, and healthy aging. Ageing Res. Rev. 39, 59–67 (2017).
Ja, W. W. et al. Prandiology of Drosophila and the CAFE assay. Proc. Natl Acad. Sci. USA 104, 8253–8256 (2007).
Murphy, K. R., Park, J. H., Huber, R. & Ja, W. W. Simultaneous measurement of sleep and feeding in individual Drosophila. Nat. Protoc. 12, 2355–2366 (2017).
Partridge, L., Alic, N., Bjedov, I. & Piper, M. D. W. Ageing in Drosophila: the role of the insulin/Igf and TOR signalling network. Exp. Gerontol. 46, 376–381 (2011).
Cox, K. H. & Takahashi, J. S. Circadian clock genes and the transcriptional architecture of the clock mechanism. J. Mol. Endocrinol. 63, R93–R102 (2019).
Dunlap, J. C. & Loros, J. J. Making time: conservation of biological clocks from fungi to animals. Microbiol Spectr. 5, FUNK-0039-2016 (2017).
Allada, R. & Chung, B. Y. Circadian organization of behavior and physiology in Drosophila. Annu. Rev. Physiol. 72, 605–624 (2010).
Panda, S. Circadian physiology of metabolism. Science 354, 1008–1015 (2016).
Duong, H. A., Robles, M. S., Knutti, D. & Weitz, C. J. A molecular mechanism for circadian clock negative feedback. Science 332, 1436–1439 (2011).
Xu, K., Zheng, X. & Sehgal, A. Regulation of feeding and metabolism by neuronal and peripheral clocks in Drosophila. Cell Metab. 8, 289–300 (2008).
García-Gaytán, A. C. et al. Synchronization of the circadian clock by time-restricted feeding with progressive increasing calorie intake. Resemblances and differences regarding a sustained hypocaloric restriction. Sci. Rep. 10, 10036 (2020).
Kinouchi, K. et al. Fasting imparts a switch to alternative daily pathways in liver and muscle. Cell Rep. 25, 3299–3314.e6 (2018).
Wang, H. et al. Time-restricted feeding shifts the skin circadian clock and alters UVB-induced DNA damage. Cell Rep. 20, 1061–1072 (2017).
Yamamuro, D. et al. Peripheral circadian rhythms in the liver and white adipose tissue of mice are attenuated by constant light and restored by time-restricted feeding. PLoS ONE 15, e0234439 (2020).
Delventhal, R. et al. Dissection of central clock function in Drosophila through cell-specific CRISPR-mediated clock gene disruption. eLife 8, e48308 (2019).
Ulgherait, M. et al. Circadian regulation of mitochondrial uncoupling and lifespan. Nat. Commun. 11, 1927 (2020).
Ulgherait, M. et al. Dietary restriction extends the lifespan of circadian mutants tim and per. Cell Metab. 24, 763–764 (2016).
Hansen, M., Rubinsztein, D. C. & Walker, D. W. Autophagy as a promoter of longevity: insights from model organisms. Nat. Rev. Mol. Cell Biol. 19, 579–593 (2018).
Scott, R. C., Schuldiner, O. & Neufeld, T. P. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev. Cell 7, 167–178 (2004).
Kalfalah, F. et al. Crosstalk of clock gene expression and autophagy in aging. Aging 8, 1876–1895 (2016).
Ma, D., Li, S., Molusky, M. M. & Lin, J. D. Circadian autophagy rhythm: a link between clock and metabolism? Trends Endocrinol. Metab. 23, 319–325 (2012).
Rubinsztein, D. C., Mariño, G. & Kroemer, G. Autophagy and aging. Cell 146, 682–695 (2011).
Chang, J. T., Kumsta, C., Hellman, A. B., Adams, L. M. & Hansen, M. Spatiotemporal regulation of autophagy during Caenorhabditis elegans aging. eLife 6, e18459 (2017).
DeVorkin, L. & Gorski, S. M. Monitoring autophagy in Drosophila using fluorescent reporters in the UAS-GAL4 system. Cold Spring Harb. Protoc. 2014, 967–972 (2014).
Ulgherait, M., Rana, A., Rera, M., Graniel, J. & Walker, D. W. AMPK modulates tissue and organismal aging in a non-cell-autonomous manner. Cell Rep. 8, 1767–1780 (2014).
Mirouse, V., Swick, L. L., Kazgan, N., St Johnston, D. & Brenman, J. E. LKB1 and AMPK maintain epithelial cell polarity under energetic stress. J. Cell Biol. 177, 387–392 (2007).
Barcelo, H. & Stewart, M. J. Altering Drosophila S6 kinase activity is consistent with a role for S6 kinase in growth. Genesis 34, 83–85 (2002).
Tricoire, H. et al. The steroid hormone receptor EcR finely modulates Drosophila lifespan during adulthood in a sex-specific manner. Mech. Ageing Dev. 130, 547–552 (2009).
Kaneko, M., Park, J. H., Cheng, Y., Hardin, P. E. & Hall, J. C. Disruption of synaptic transmission or clock-gene-product oscillations in circadian pacemaker cells of Drosophila cause abnormal behavioral rhythms. J. Neurobiol. 43, 207–233 (2000).
Plautz, J. D., Kaneko, M., Hall, J. C. & Kay, S. A. Independent photoreceptive circadian clocks throughout Drosophila. Science 278, 1632–1635 (1997).
Duffy, J. F., Zitting, K.-M. & Chinoy, E. D. Aging and circadian rhythms. Sleep Med. Clin. 10, 423–434 (2015).
Cabrera, D., Young, M. W. & Axelrod, S. Time-restricted feeding prolongs lifespan in Drosophila in a peripheral clock-dependent manner. Preprint at bioRxiv https://doi.org/10.1101/2020.09.14.296368 (2020).
Delventhal, R. et al. Dissection of central clock function in Drosophila through cell-specific CRISPR-mediated clock gene disruption. eLife 8, e48308 (2019).
Murphy, K. R. et al. Postprandial sleep mechanics in Drosophila. eLife 5, e19334 (2016).
Rera, M., Clark, R. I. & Walker, D. W. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc. Natl Acad. Sci. USA 109, 21528-21533 (2012).
Rera, M. et al. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metab. 14, 623-634 (2011).
Hughes, M. E., Hogenesch, J. B. & Kornacker, K. JTK_CYCLE: an efficient nonparametric algorithm for detecting rhythmic components in genome-scale data sets. J. Biol. Rhythms 25, 372-380 (2010).
Claesson, M. J. et al. Comparison of two next-generation sequencing technologies for resolving highly complex microbiota composition using tandem variable 16S rRNA gene regions. Nucleic Acids Res. 38, e200 (2010).
Rana, A., Rera, M. & Walker, D. W. Parkin overexpression during aging reduces proteotoxicity, alters mitochondrial dynamics, and extends lifespan. Proc. Natl Acad. Sci. USA 110, 8638-8643 (2013).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671-675 (2012).
Copeland, J. M. et al. Extension of Drosophila life span by RNAi of the mitochondrial respiratory chain. Curr. Biol. 19, 1591-1598 (2009).
Acknowledgements
We thank all members of the Shirasu-Hiza and Canman laboratories for support, discussions and feedback. We also thank D. W. Walker and J. Giebultowicz for fly lines. Other stocks were obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537). Work was supported by Charles H. Revson Foundation (to M.U.), AFAR Glenn Foundation Postdoctoral Fellowship for Aging Research (to M.U.), Celia Lipton Farris and Victor W. Farris Foundation Graduate Student Fellowship (to S.J.P.), NIH T32GM007088 (to J.G.), NIH R01GM117407 (to J.C.C.), NIH R01GM130764 (to J.C.C.), Joe W. and Dorothy Dorsett Brown Foundation (to W.W.J.), NIH R56AG065986 (to W.W.J.), NIH R35GM127049 (to M.S.-H.) and NIH R01AG045842 (to M.S.-H.).
Author information
Authors and Affiliations
Contributions
M.U. and M.S.-H. conceived the experiments. Experiments were performed and analysed by M.U. (all), A.M.M. (lifespan experiments), S.J.P. and W.W.J. (feeding analysis), J.G. (western blots and lifespan experiments), S.J.T. (qRT–PCR and lifespan experiments), J.S. (lifespan experiments) and N.K. (biochemical and imaging experiments, and western blots). M.U., J.C.C. and M.S.-H. made intellectual contributions, designed the figures and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Tor Erik Rusten and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
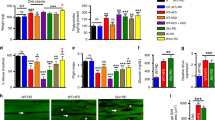
Extended Data Fig. 1 Lifespan changes in response to different feeding and fasting regimens.
Light blue boxes on graphs indicate duration of TRF (aqua), IF (medium blue), or iTRF (sky blue) during lifespan. a, Schematic of different feeding regimens utilized in Drosophila lifespan screen. b, 12-hour time-restricted feeding (TRF) from day 10 until death shortened female lifespan (top; ad lib, solid gray, n=292; TRF, dashed gray, n=142) and minimally extended male lifespan (bottom; ad lib, solid, n=241; TRF, dashed, n=314). c, In contrast, TRF from days 10-40 extended female (top; ad lib, solid gray, n=292; TRF, dashed gray, n=150) and male (bottom; ad lib, solid gray, n=241; TRF, dashed gray, n=406) lifespan. d, 24-hour intermittent fasting (IF) from day 10-death shortened both female (top; ad lib, solid gray, n=145; IF, dashed gray, n=149) and male (bottom top; ad lib, solid gray, n=241; IF, dashed gray, n=276) lifespan. e, Intermittent time-restricted feeding (iTRF) from day 10-death did not extend lifespan (ad lib, solid gray, n=142; TRF, dashed gray, n=157). f, iTRF regimen from days 10-40 extended male lifespan (ad lib, solid gray, n=323; TRF, dashed gray, n=382). (See Methods and SI for trials, statistics, and source data; n=number of individual flies; p-values were obtained by log-rank analysis (b–f).
Extended Data Fig. 2 Characterization of iTRF windows and effect on feeding and dietary restriction.
Light blue boxes on graphs indicate duration of iTRF during lifespan; solid and dashed lines represent flies on ad lib and iTRF diets, respectively. a–e, 10-day periods of iTRF in early to mid-life (days 10-40 of adulthood) can extend lifespan but not later in life (days 40-50): (a) days 10-20 with females (ad lib n=311; iTRF n=319); days 20-30 with (b) females (iTRF n=337) and (c) males (ad lib n=323; iTRF n=366); days 30-40 with (d) females (iTRF n=355) and (e) males (iTRF n=293) all extend lifespan. f, g, iTRF days 40-50 of adulthood did not extend male (iTRF n=302) or female (iTRF n=349) lifespan. h, Relative to flies on ad lib diet (dark gray dots, n=13), flies on iTRF (shown as blue or green dots depending on diet phase, n=14) starve during the fasting phase (n.a., no food available) and eat more during the feeding phase (green dots). i, iTRF extended mean lifespan regardless of dietary protein concentration (n=98-347 for each sample of ad lib or iTRF flies at each protein concentration). j, After partial genetic ablation of insulin producing cells, iTRF still extended lifespan (dashed brown, n=424) relative to ad lib diet (solid brown, n=310), to a similar extent as in genetic controls (ad lib, solid gray, n=161; iTRF, dashed gray, n=180). (See Methods and SI for trials, statistics, and source data; n=number of individual flies; ****=p<0.0001; p-values were obtained by log-rank analysis (a-g, and j) and unpaired two-tailed t-test (h-i). Center values=averages; error bars=SEM.).
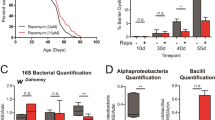
Extended Data Fig. 3 iTRF delays aging markers (protein aggregation and intestinal dysfunction) and extends lifespan independent of microbiota.
a, Representative western blot of Triton-insoluble protein accumulation of p62/ref(2)P (each sample=30 flies/condition/timepoint; see also Supplementary Fig. 1). b, Quantification of triton insoluble protein levels showed that iTRF flies (light gray) exhibited reduced accumulation of p62/ref(2)P with age, relative to ad lib flies (dark gray) (average of 4 biological repeats). c, Representative images of 40-day old indirect flight muscle stained for polyubiquitin protein aggregates (green), p62/ref(2)P (magenta), and filamentous actin (F-actin, blue); scale bar=50 µm. d, e, iTRF significantly reduced (d) polyubiquitin aggregates and (e) accumulation of p62 aggregates (ad lib n=10 thoraces, iTRF n=11 thoraces). f, iTRF also reduced age-related intestinal over-proliferation, as marked by phospho-histone H3 staining (p-HH3) (ad lib n=8 guts; iTRF n=9 guts); scale bar=50 µm. g, Light blue boxes on graphs indicate duration of iTRF during lifespan. iTRF (dashed line) delayed age-related intestinal barrier dysfunction relative to ad lib (solid line), as marked by decreased numbers of smurf flies (n=8-12 cohorts of 20-31 flies). h–j, Light colored boxes on graphs indicate duration of antibiotic treatment (AB, green) or antibiotic treatment plus iTRF diet (blue/green striped) during lifespan. iTRF flies showed delayed age-related growth in microbiome load with age (n=30 flies/condition/timepoint, 4 biological replicates) (h). iTRF extended lifespan upon microbiome clearance via antibiotics treatment during either (i) total lifespan (ad lib n=227, iTRF n=268) or (j) only days 10-40 of adulthood (ad lib n=144, iTRF n=190). (See Methods and SI for trials, statistics, and source data; n=number of flies unless otherwise indicated; n.s.=p>0.05, *=p<0.05, **=p<0.01, ***=p<0.001, ****=p<0.0001; p-values were obtained by ANOVA followed by Tukey’s post-hoc test (b, g, h), unpaired two-tailed student’s t-test (d–f), and log-rank analysis (i, j). Center values=averages; error bars=SEM.).
Extended Data Fig. 4 Circadian mutants show a normal lifespan response to dietary protein restriction but do not respond to iTRF.
a, Gene expression of timeless, similar to period and Clock, was enhanced by iTRF during the fasting phase (each n=4 biological replicates of 30 female flies/genotype/condition/timepoint; unmarked=n.s.). b, c, Light blue boxes on graphs indicate duration of iTRF during lifespan. Circadian mutants did not respond to iTRF with extended lifespan relative to controls (ad lib n=187-288; iTRF n=290-311): (b) cycle01 (ad lib n=65, iTRF n=121) and (c) timeless01 (ad lib n=120, iTRF n=152) and period01 (ad lib n=215, iTRF n=184) null mutant females did not respond to iTRF with extended lifespan. d, e, (d) cycle01 and (e) period01 mutant females showed a normal “tent-curve” lifespan response to dietary protein titration (n=61-272 flies/genotype/condition/timepoint). f, period01 mutant females did not starve significantly faster than controls whether they have been on iTRF or ad lib diet (controls: ad lib n=31, iTRF n=35; per: ad lib n=27, iTRF n=42). g, Similar to controls (gray, ad lib n=30; light gray, iTRF, n=29), period01 mutant females (orange, ad lib n=27; light orange, iTRF, n=27) ate more on iTRF relative to on ad lib diet. h, Unlike control iTRF flies (light gray), which had reduced accumulation of p62/ref(2)P with age relative to ad lib flies (dark gray), per mutants had similar levels on ad lib (orange) or iTRF (light orange) diets (each dot=1 sample=30 flies; each bar=average of 4 biological repeats). Actin blot is repeated from Fig. 2h because the same western blot was used to quantify Ubiquitin, p62/ref(2)P, and actin (loading control); see also Supplementary Fig. 1. i, Representative images of indirect flight muscle from 40-day-old flies stained for filamentous actin (F-actin, blue), ubiquitin (green), and p62/ref(2)P (magenta) showed that, unlike genetic controls (ad lib n=10, iTRF n=11 thoraces), period mutants did not have decreased polyubiquitin, or p62/ref(2)P aggregate accumulation in response to iTRF (ad lib n=10, iTRF n=10 thoraces); scale bar=50 µm. (See Methods and SI for trials, statistics, and source data; n=number of flies unless otherwise indicated; n.s.=p>0.05, *=p<0.05, **=p<0.01, ***=p<0.001, ****=p<0.0001; p-values were obtained by ANOVA followed by Tukey’s post-hoc test (a, g–i) and log-rank analysis (b, c, f). Center values=averages; error bars=SEM.).
Extended Data Fig. 5 period mutants are defective in autophagy regulation and autophagy induction in response to fasting.
a, b, Similar to circadian genes, iTRF increased the peak amplitude of (a) atg1 and (b) atg8a mRNA expression during the fasting period in wild-type flies (gray) but not period01 mutants (orange) (each dot=1 sample of 30 flies; each bar=average of 4 biological repeats). c, d, period01 mutants (orange) had (c) reduced activation of AMPK and (d) high levels of TORC1 activity as marked by S6K phosphorylation, both in response to fasting during iTRF, compared to controls (gray) (each dot=1 sample of 30 flies; each bar=average of 4 biological repeats); see also Extended Data Fig. 10. e, Representative images of posterior midgut cells during fasting phase of iTRF of 35-day-old flies labeled with LysoTracker™ (magenta), GFP-Atg8a (green), and DAPI to label the DNA (blue), showed that control animals (n=8 guts; each dot represents 2-3 Z-stacks of the posterior midgut of 1 animal) had high levels of LysoTracker™ and GFP-Atg8a puncta compared to period mutants (n=8 guts); scale bar=20 µm; white dashed boxes on images represent inset area presented in Fig. 3g. (See Methods and SI for trials, statistics, and source data; n=number of flies unless otherwise indicated; n.s.=p>0.05, *=p<0.05, **=p<0.01, ***=p<0.001, ****=p<0.0001; p-values were obtained by ANOVA followed by Tukey’s post-hoc test (a-d) and unpaired, two-tailed t-test (e). Center values=averages; error bars=SEM.).
Extended Data Fig. 6 Circadian manipulation of upstream metabolic and autophagy regulators partially determines lifespan response to iTRF.
Light blue boxes on graphs indicate duration of iTRF during lifespan. a–d, Relative to controls (gray: ad lib, solid, n=161; iTRF, dashed, n=164), which had an ~20% increase in mean lifespan in response to iTRF, circadian overexpression of: (a) dominant-negative (DN) AMPK (K57A, sage green) shortened the lifespan of animals on ad lib diets (solid, n=184) and caused a 13% increase in mean lifespan in response to iTRF (dashed, n=170); (b) constitutively active (CA) AMPK (T184D, dark green) extended lifespan on ad lib diet (solid, n=156) and caused an 8% increase in mean lifespan in response to iTRF (dashed, n=134); (c) dominant-negative (DN) S6K (KQ, light blue) extended lifespan on ad lib diet (solid, n=292) and caused a 12% increase in mean lifespan in response to iTRF (dashed, n=180); (d) constitutively active CA-S6K (STDETE, medium blue) minimally shortened lifespan on ad lib diets (solid, n=237) and caused an 8% increase in mean lifespan in response to iTRF (dashed, n=282). e, f, RU486 feeding did not influence control (e) or per01 (f) lifespan in flies lacking UAS transgenes (control: ad lib n=136-146, iTRF n=129-142; per01: ad lib n=294-501, iTRF n=238-415). (See Methods and SI for trials, statistics, and source data; n=number of flies; p-values were obtained by log-rank analysis (a–f)).
Extended Data Fig. 7 Circadian regulation of Atg8a is necessary for iTRF and sufficient to extend lifespan.
a, Using tim-GAL4 to drive expression of mCherry-atg8a, we confirmed oscillating mCherry-Atg8a and free mCherry protein expression by western blot analysis (see also Supplementary Fig. 1), which demonstrated circadian autophagic flux in controls on ad lib diet (each lane=30 flies; each time point of quantification=average of 3 biological repeats). b–d, Solid lines represent ad lib flies; dashed lines represent iTRF flies; light blue boxes on graphs indicate duration of iTRF during lifespan. RNAi-mediated circadian knockdown of (b) atg1 (pink: ad lib n=217, iTRF n=166) and (c) atg8a (purple: ad lib n=196, iTRF n=139) was necessary for iTRF-mediated lifespan extension (controls, gray: ad lib n=194-316, iTRF n=196-409. (d) Circadian overexpression of mCherry-Atg8a was sufficient to extend lifespan on ad lib diet (solid lines: gray, control n=185; purple, mCh-atg8a n=422) and responded minimally to iTRF (dashed lines: gray, control n=421, purple, mCh-atg8a n=437). (See Methods and SI for trials, statistics, and source data; n=number of flies unless otherwise indicated; p-values were obtained by log-rank analysis (b–d). Center values=averages; error bars=SEM.).
Extended Data Fig. 8 atg1 is necessary and sufficient for iTRF-mediated delays in aging-associated climbing defects and protein aggregation.
a, c, Light blue boxes on graphs indicate duration of iTRF during lifespan. a, b, Relative to controls (gray), circadian knockdown of atg1 (pink) increased aging markers of (a) climbing defects (n=10 vials of 10 flies/condition/genotype/timepoint) and (b) protein aggregation (each lane=30 flies; each time point of quantification=average of 4 biological repeats) and made flies resistant to the effects of iTRF (dashed lines, lighter shades), relative to ad lib diets (solid lines, darker shades). c, d, In contrast, relative to controls (gray), circadian overexpression of atg1 (magenta) decreased aging markers of (c) climbing defects (n=10 vials of 10 flies/condition/genotype/timepoint) and (d) protein aggregation (each lane=30 flies; each time point of quantification=average of 4 biological repeats; see also Supplementary Fig. 1) and also made flies resistant to the effects of iTRF (dashed lines), relative to ad lib diets (solid lines). (See Methods and SI for trials, statistics, and source data; n=number of flies unless otherwise indicated; n.s.=p>0.05, ***=p<0.001, ****=p<0.0001; p-values were obtained by ANOVA followed by Tukey’s post-hoc test (a–d). Center values=averages; error bars=SEM.).
Extended Data Fig. 9 Enhanced autophagy specifically during the night phase is necessary and sufficient for TRF-mediated lifespan extension.
a, RU-induced overexpression of atg1 during iTRF-like phases of the circadian cycle causes circadian enhanced, night-specific expression of atg1 (n=4 biological replicates of 30 flies/timepoint/condition; unmarked=n.s.). b, c, Light aqua boxes on graphs indicate duration of shifted-TRF during lifespan. b, Relative to ad lib diet (period01 mutants ad lib, solid orange, n=225), neither night-biased 12:12 treatment of shifted TRF (dashed orange, n=228) or RU-induced atg1 expression (solid magenta, n=319) alone extended the lifespan of per01 mutants. Combined, night-biased 12:12 shifted TRF and RU-induced atg1 expression modestly increased lifespan of per01 mutants (dashed magenta, n=239). c, Replotted here are per01 mutants on ad lib diet (solid orange, n=225) and on night-biased 12:12 shifted TRF (dashed orange, n=228). Day-biased 12:12 RU-induced exogenous atg1 expression decreased the lifespan of per01 mutants (solid magenta, n=206); this lifespan was increased by night-biased shifted TRF (dashed magenta, n=192). Also shown below are re-plots comparing control and per01 mutant backgrounds with night (b) and day (c) biased RU-induced atg1 expression on ad lib diet (second row) or shifted TRF (third row). d, Graphic schematic illustrating endogenous rhythms of atg1 expression (gray) and the predicted effects of RU treatment and 12:12 TRF, either night biased and day-biased, on exogenous atg1 expression. (See Methods and SI for trials, statistics, and source data; n=number of flies unless otherwise indicated; n.s.=p>0.05, ***=p<0.001, ****=p<0.0001; p-values were obtained by ANOVA followed by Tukey’s post-hoc test (a) and log-rank analysis (b, c). Center values=averages; error bars=SEM.).
Supplementary information
41586_2021_3934_MOESM1_ESM.pdf
This file contains the Western blots used in this article Dashed orange boxes indicate region of blot shown in each figure. (a) Blots in Figure 1f and Extended Data Figure 3a showing anti-Ubiquitin (Ub, top), anti-p62 (ref(2)P; center), and anti-Actin (bottom) western blot staining of ad lib or iTRF fed-flies at days 7 or 40 post-eclosion. (b) Blots in Figure 2h and Extended Data Figure 4h showing anti-Ubiquitin (Ub, top), anti-p62 (ref(2)P; center), and anti-Actin (bottom) western blot staining of control and period mutant flies fed ad lib or iTRF diets at days 7 or 40 post-eclosion. (c) Blots in Figure 3e showing anti-mCherry (top) and anti-Actin (bottom) western blot staining of control and period mutant flies. (d) Blots in Extended Data Figure 7a showing anti-mCherry (top) and anti-Actin (bottom) western blot staining of control and timeless-GAL4 driven mCherry-atg8a expressing flies. (e) Blots in Extended Data Figure 8b showing anti-Ubiquitin (Ub, top), anti-p62 (ref(2)P; center), and anti-Actin (bottom) western blot staining of control and atg1-RNAi flies fed ad lib or iTRF diets at days 7 or 40 post-eclosion. (f) Blots in Extended Data Figure 8d showing anti-Ubiquitin (Ub, top), anti-p62 (ref(2)P; center), and anti-Actin (bottom) western blot staining of control and Atg1-overexpressing flies fed ad lib or iTRF diets at days 7 or 40 post-eclosion.
Supplementary Table 1
Supplementary Table 1 contains exact n, mean and medians, number of trials, and statistics for all lifespan experiments (Figures 1-4, Extended Data Figures 1-4 and 6-9), as well as climbing assays (Figures 2g, 8a, and 8c). Repeats are listed as “rep” in the “Figure” column; LS = lifespan; n=number of individual flies; p-values were obtained by log-rank analysis.
Supplementary Table 2
Supplementary Table 2 is the raw data source file, composed of a spreadsheet with 13 tabs; each tab contains the source data for a figure (Fig. 1-4) or extended data figure (ED1-9). Source data include tables of subjects at risk for lifespans, average food intake as measured by CaFe assay, percent flies past the marker for climbing assays, calculated mRNA levels using standard qRT-PCR methods, and blot quantitations.
Rights and permissions
About this article
Cite this article
Ulgherait, M., Midoun, A.M., Park, S.J. et al. Circadian autophagy drives iTRF-mediated longevity. Nature 598, 353–358 (2021). https://doi.org/10.1038/s41586-021-03934-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03934-0
This article is cited by
-
Neuronal knockdown of Cullin3 as a Drosophila model of autism spectrum disorder
Scientific Reports (2024)
-
Six-hour time-restricted feeding inhibits lung cancer progression and reshapes circadian metabolism
BMC Medicine (2023)
-
Cellular rejuvenation: molecular mechanisms and potential therapeutic interventions for diseases
Signal Transduction and Targeted Therapy (2023)
-
The hunger strikes back: an epigenetic memory for autophagy
Cell Death & Differentiation (2023)
-
Early-onset caloric restriction alleviates ageing-associated steatohepatitis in male mice via restoring mitochondrial homeostasis
Biogerontology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.