Abstract
Metastasis is the leading cause of cancer-related death in humans. It is a complex multistep process during which individual tumour cells spread primarily through the circulatory system to colonize distant organs1,2,3. Once in the circulation, tumour cells remain vulnerable, and their metastatic potential largely depends on a rapid and efficient way to escape from the blood stream by passing the endothelial barrier4,5,6,7,8,9. Evidence has been provided that tumour cell extravasation resembles leukocyte transendothelial migration7,8,9. However, it remains unclear how tumour cells interact with endothelial cells during extravasation and how these processes are regulated on a molecular level. Here we show that human and murine tumour cells induce programmed necrosis (necroptosis) of endothelial cells, which promotes tumour cell extravasation and metastasis. Treatment of mice with the receptor-interacting serine/threonine-protein kinase 1 (RIPK1)-inhibitor necrostatin-1 or endothelial-cell-specific deletion of RIPK3 reduced tumour-cell-induced endothelial necroptosis, tumour cell extravasation and metastasis. In contrast, pharmacological caspase inhibition or endothelial-cell-specific loss of caspase-8 promoted these processes. We furthermore show in vitro and in vivo that tumour-cell-induced endothelial necroptosis leading to extravasation and metastasis requires amyloid precursor protein expressed by tumour cells and its receptor, death receptor 6 (DR6), on endothelial cells as the primary mediators of these effects. Our data identify a new mechanism underlying tumour cell extravasation and metastasis, and suggest endothelial DR6-mediated necroptotic signalling pathways as targets for anti-metastatic therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Valastyan, S. & Weinberg, R. A. Tumor metastasis: molecular insights and evolving paradigms. Cell 147, 275–292 (2011)
Wan, L., Pantel, K. & Kang, Y. Tumor metastasis: moving new biological insights into the clinic. Nature Med. 19, 1450–1464 (2013)
Vanharanta, S. & Massagué, J. Origins of metastatic traits. Cancer Cell 24, 410–421 (2013)
Bos, P. D. et al. Genes that mediate breast cancer metastasis to the brain. Nature 459, 1005–1009 (2009)
Padua, D. et al. TGFβ primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell 133, 66–77 (2008)
Gupta, G. P. et al. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature 446, 765–770 (2007)
Reymond, N., d’Água, B. B. & Ridley, A. J. Crossing the endothelial barrier during metastasis. Nature Rev. Cancer 13, 858–870 (2013)
Labelle, M. & Hynes, R. O. The initial hours of metastasis: the importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discov . 2, 1091–1099 (2012)
Joyce, J. A. & Pollard, J. W. Microenvironmental regulation of metastasis. Nature Rev. Cancer 9, 239–252 (2009)
Galluzzi, L. et al. Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ. 22, 58–73 (2015)
Pasparakis, M. & Vandenabeele, P. Necroptosis and its role in inflammation. Nature 517, 311–320 (2015)
Zhou, W. & Yuan, J. Necroptosis in health and diseases. Semin. Cell Dev. Biol. 35, 14–23 (2014)
Silke, J., Rickard, J. A. & Gerlic, M. The diverse role of RIP kinases in necroptosis and inflammation. Nature Immunol. 16, 689–697 (2015)
Oberst, A. et al. Catalytic activity of the caspase-8–FLIPL complex inhibits RIPK3-dependent necrosis. Nature 471, 363–367 (2011)
Kaiser, W. J. et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471, 368–372 (2011)
Krysko, D. V., Vanden Berghe, T., D’Herde, K. & Vandenabeele, P. Apoptosis and necrosis: detection, discrimination and phagocytosis. Methods 44, 205–221 (2008)
Pan, G. et al. Identification and functional characterization of DR6, a novel death domain-containing TNF receptor. FEBS Lett. 431, 351–356 (1998)
Lavrik, I., Golks, A. & Krammer, P. H. Death receptor signaling. J. Cell Sci. 118, 265–267 (2005)
Liu, J. et al. Enhanced CD4+ T cell proliferation and Th2 cytokine production in DR6-deficient mice. Immunity 15, 23–34 (2001)
Huang, G. et al. Death receptor 6 (DR6) antagonist antibody is neuroprotective in the mouse SOD1G93A model of amyotrophic lateral sclerosis. Cell Death Disease 4, e841 (2013)
Nikolaev, A., McLaughlin, T., O’Leary, D. D. M. & Tessier-Lavigne, M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature 457, 981–989 (2009)
Xu, K., Olsen, O., Tzvetkova-Robev, D., Tessier-Lavigne, M. & Nikolov, D. B. The crystal structure of DR6 in complex with the amyloid precursor protein provides insight into death receptor activation. Genes Dev. 29, 785–790 (2015)
Takagi, K. et al. Amyloid precursor protein in human breast cancer: an androgen-induced gene associated with cell proliferation. Cancer Sci. 104, 1532–1538 (2013)
Takayama, K. et al. Amyloid precursor protein is a primary androgen target gene that promotes prostate cancer growth. Cancer Res. 69, 137–142 (2009)
Yang, Z., Fan, Y., Deng, Z., Wu, B. & Zheng, Q. Amyloid precursor protein as a potential marker of malignancy and prognosis in papillary thyroid carcinoma. Oncol. Lett. 3, 1227–1230 (2012)
Hick, M. et al. Acute function of secreted amyloid precursor protein fragment APPsα in synaptic plasticity. Acta Neuropathol. 129, 21–37 (2015)
O’Reilly, L. A. et al. Membrane-bound Fas ligand only is essential for Fas-induced apoptosis. Nature 461, 659–663 (2009)
Kaczmarek, A., Vandenabeele, P. & Krysko, D. V. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity 38, 209–223 (2013)
Takai, E. et al. Autocrine regulation of TGF-β1-induced cell migration by exocytosis of ATP and activation of P2 receptors in human lung cancer cells. J. Cell Sci. 125, 5051–5060 (2012)
Schumacher, D., Strilic, B., Sivaraj, K. K., Wettschureck, N. & Offermanns, S. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell 24, 130–137 (2013)
Liang, C. C., Park, A. Y. & Guan, J. L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nature Protocols 2, 329–333 (2007)
Korhonen, H. et al. Anaphylactic shock depends on endothelial Gq/G11. J. Exp. Med. 206, 411–420 (2009)
Cermak, T. et al. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 39, e82 (2011)
Dittgen, T. et al. Lentivirus-based genetic manipulations of cortical neurons and their optical and electrophysiological monitoring in vivo. Proc. Natl Acad. Sci. USA 101, 18206–18211 (2004)
Young-Pearse, T. L. et al. A critical function for β-amyloid precursor protein in neuronal migration revealed by in utero RNA interference. J. Neurosci. 27, 14459–14469 (2007)
Sivaraj, K. K. et al. G13 controls angiogenesis through regulation of VEGFR-2 expression. Dev. Cell 25, 427–434 (2013)
Acknowledgements
We thank S. Fulda and our friends and colleagues for comments on the manuscript. We also thank S. Hümmer for secretarial help and C. Ringel, J. Hoffmann, I.-M. Gross, D. Magalei and M. Winkels for technical help. This work was supported by the German Cancer Aid and the Max Planck Society. K.H. was supported by the China Scholarship Council. U.C.M. was supported by a grant from the Deutsche Forschungsgemeinschaft (MU 1457/9-2). M.P. received funding from the European Research Council (grant agreement 323040), the Deutsche Forschungsgemeinschaft (SFB670, SFB829), Worldwide Cancer Research (grant 15-0228) and the Helmholtz Alliance Preclinical Comprehensive Cancer Center.
Author information
Authors and Affiliations
Contributions
B.S. performed most of the experiments and analysed the data. L.Y. generated mice with a conditional Ripk3 allele and contributed to in vitro and in vivo studies. J.A.J. contributed to in vitro experiments. L.W. and M.P. generated MLKL-deficient animals. K.H. and U.C.M. purified APPsα and performed APP-related experiments. B.S. and S.O. designed the study, discussed data and wrote the manuscript. All authors commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks C. Betsholtz, S. Tavazoie and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
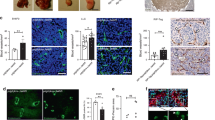
Extended Data Figure 1 TCs induce necroptosis in ECs.
a, Criteria for the discrimination of apoptotic and necrotic EC death. Fluorescent images of HUVECs cultured under conditions to induce apoptosis (TRAIL, Staurosporine) or to induce necrosis (H2O2, hypoxia (1% O2)) and stained with Hoechst 33342 (blue) and the membrane-impermeable dye ethidium homodimer III (EthD-III, red). Asterisks indicate apoptotic ECs (condensed, fragmented nuclei, EthD-III-negative); closed arrowheads indicate necrotic ECs (normal nuclei, EthD-III-positive). Late apoptotic cells are indicated by open arrowheads (condensed/fragmented nuclei, EthD-III-positive); scale bar, 5 μm. b, c, No annexin-V-positive cells were detected in HUVECs cultured in the presence of TCs (MDA-MB-231). Stimulation with TNF-α served as positive control; scale bar, 20 μm. d, Fluorescent images of L929 cells stimulated with TNF-α to induce programmed necrosis (necroptosis, arrowheads). This effect was reversed when cells were additionally cultured with the RIPK1 inhibitor necrostatin-1 (Nec-1); scale bar, 10 μm. e, Effect of freshly isolated PBMCs on EC necrosis either directly or in the presence of MDA-MB-231 TCs. PBMCs contained 20 times the amount of platelets (that is, 3 × 104, 9 × 104 or 3 × 105 platelets). f–h, Quantification of necrosis in HMVEC-L (f), in freshly isolated primary mouse lung ECs (prim. MLEC) (g) or in HUVEC (h) cultured in the presence of different human and mouse TCs (TCs) and at different concentrations as indicated. i, Representative confocal images of HUVEC cultured in the absence of TCs (-TC) or presence of TCs (+TC, green) and stained as indicated; scale bar, 5 μm. Quantification of EthD-III- and phospho (p)-MLKL-double-positive ECs (more than 50 EthDIII-positive cells were analysed). j, k, Analysis of knockdown efficiencies in HUVEC by western blot for RIPK3 and caspase-8 (j) or by quantitative RT–PCR for MLKL (k). α-Tubulin served as loading control in j and relative mRNA expression levels normalized to GAPDH and to the level detected in scramble siRNA-treated samples (siCTRL) are shown in k. Shown are representative data of two (c, g) or three (e, f, h) independent experiments with mean values ± s.e.m. from biological sextuplicates (n = 6). *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant. One-way ANOVA and Bonferroni’s post hoc test (c, e, g, h) or unpaired, two-tailed Student’s t-test (f). For gel source data see Supplementary Fig. 1.
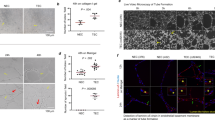
Extended Data Figure 2 TCs induce necroptosis in ECs in vivo.
a, Quantitative evaluation of cleaved caspase-3- and EthD-III-positive cells in lungs of C57BL/6 WT animals at the indicated time points after i.v. injection of B16 TCs on the basis of the analysis of confocal microscopy images as shown in main Fig. 2a, b. TCs were injected at the same time and lung isolation occurred at the indicated time points. Injection of PBS served as control. b, Quantification of EthD-III-positive ECs in lungs of WT animals 6 h after i.v. injection of B16 or LLC1 TCs. c–e, Representative confocal images of lung sections taken 6 h after i.v. injection of B16 or LLC1 TCs (c, d) or injection of equal amounts of fluorescently labelled 15 μm microspheres (e) into WT animals and stained for the indicated markers. Arrowheads in c indicate EthD-III-positive cells. Isolated lungs from animals cultured ex vivo in the presence of staurosporine served as positive controls in d. Scale bar, 20 μm. Shown are representative data of two (a) or three (b) independent experiments with mean values ± s.e.m. from n = 3 animals per time point (a) or n = 6 animals per group (b). **P < 0.01; ***P < 0.001. One-way ANOVA and Bonferroni’s post hoc test.
Extended Data Figure 3 Targeting strategy for the generation of mice with loxP-flanked (floxed (fl)) Ripk3 allele.
a, Targeting scheme for the generation of floxed RIPK3 including 3′ Southern screening for the identification of positive ES cell clones and 3′ and 5′ Southern blot confirmation of heterozygous and homozygous floxed Ripk3 alleles, respectively. b, Western blot analysis of RIPK3 and VE-cadherin (VE-Cad) in primary ECs isolated from lungs (MLEC) of tamoxifen-treated Tie2-CreERT2;RIPK3loxP/loxP animals (RIPK3ECKO). Cre-negative littermates served as control. α-tubulin served as loading control. c, Quantification of Evans blue permeability in the lungs of RIPK3ECKO animals. d, Quantification of transmigrated B16 or LLC1 TCs over a layer of DMSO- or 4-OH-tamoxifen-treated primary MLEC isolated from uninduced Tie2-CreERT2;RIPK3loxP/loxP animals or Cre-negative control littermates. e, f, Quantification of lung metastases 12 d after i.v. injection (e) or 27 d after excision of a primary tumour induced by s.c. injection (f) of B16 or LLC1 TCs into RIPK3ECKO animals. Cre-negative littermates served as control. No significant differences in primary tumour growth were observed (data not shown). Shown are representative data of three (c–e) or two (f) independent experiments with mean values ± s.d. from n = 3 (c) or n = 6 (e) animals per group or from n = 11 (B16, control), n = 6 (B16, RIPK3ECKO), n = 8 (LLC1, control) and n = 6 (LLC1, RIPK3ECKO) animals (f) or n = 6 wells per condition (d). *P < 0.05; **P < 0.01; n.s., not significant. Unpaired, two-tailed Student’s t-test (c, e, f) or one-way ANOVA and Bonferroni’s post hoc test (d). For gel source data see Supplementary Fig. 1.
Extended Data Figure 4 Reduced TC-induced endothelial necroptosis and metastasis in MLKL−/− mice.
a, A TALEN pair targeting the indicated sequences in the second exon of the mouse Mlkl gene was cloned and transcribed into mRNA. b, Analysis of mice born after mRNA injection into fertilized oocyte injection by PCR for exon 2 (top) and subsequent sequence specific endonuclease assay (T7EI, bottom) for the detection of mutant alleles. DNA from C57BL/6 (WT) or TALEN-transfected cells (mut) served as control. c, The mutant allele of mouse 64 was further analysed using Sanger sequencing revealing a 8 bp deletion (Δ8), predicted to generate a premature stop codon. d, Spleen and lung extracts of three B6D2F1 MLKL+/+ WT and three homozygous MLKL mutant mice (MLKL−/−) were probed for Mlkl protein expression. Polyclonal MLKL antibody detected Mlkl protein only in WT extracts but also an additional non-specific band of similar size. e–i, B6D2F1 MLKL+/+ WT or MLKL−/− animals were injected i.v. with B16 TCs and lungs were analysed after 6 h for pulmonary EthD-III-positive ECs (f) and extravascular TCs (g) or after 12 d for lung metastases (i). Representative confocal images of lung sections and images of whole lungs are shown in (e, h); scale bar, 50 μm. Shown are representative data of two independent experiments with mean values ± s.e.m. (f, g) or ± s.d. (i) from n = 3 animals per condition (f, g) or n = 8 (MLKL+/+) and n = 11 (MLKL−/−) animals (i). **P < 0.01; ***P < 0.001. Unpaired, two-tailed Student’s t-test. For gel source data see Supplementary Figs 1 and 2.
Extended Data Figure 5 Effect of Nec-1 and Nec-1s treatment on metastasis formation.
a–c, Effect of Nec-1 (30 μM) on B16 TC proliferation (a), viability (b) and migration (c). d, Representative confocal images of lung sections taken 6 h and images of whole lungs taken 12 d after i.v. injection of B16 TCs into WT animals treated with DMSO (control) or Nec-1; scale bar, 50 μm. e, f, Lung metastases 12 d after i.v. injection of LLC1 TCs into WT animals treated with Nec-1 (e) or B16 TCs injected into WT animals treated with stable Nec-1s (f). DMSO served as control. g, h, Human MDA-MB-231 TCs were injected i.v. into Nec-1 treated immunodeficient SCID mice and pulmonary EthD-III-positive endothelial and lung metastases were analysed after 6 h and 12d, respectively. Arrowheads in the H&E-stained lung sections (h) indicate metastases; scale bar, 50 μm. In all metastasis experiments, animals were treated with Nec-1 or Nec-1s shortly before and at 3 h after TC injection (plus at 6 h for the 12 d experiment). Shown are representative data of three (a–c, e, g) or two (f, h) independent experiments with mean values ± s.e.m. (a, b, g) or ± s.d. (c, e, f, h) from biological sextuplicates (n = 6) (a, b), triplicates (n = 3) (c) or from n = 3 (g) or n = 6 animals per condition (e, f, h). *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant. Unpaired, two-tailed Student’s t-test (a–c, e, f, h) or one-way ANOVA and Bonferroni’s post hoc test (g).
Extended Data Figure 6 Effects of zVAD treatment and EC-specific loss of caspase–8 on metastasis formation.
a, Western blot analysis of uncleaved caspase-8 (Casp8) and VE-cadherin (VE-Cad) in primary MLECs of tamoxifen-treated Tie2-CreERT2;Casp8loxP/loxP animals (Casp8ECKO). b, Quantification of Evans blue permeability in the lungs of Casp8ECKO animals. c, Quantification of transmigrated B16 or LLC1 TCs over a layer of DMSO- or 4-OH-tamoxifen-treated primary MLEC from uninduced Tie2-CreERT2;Casp8loxP/loxP animals or Cre-negative control littermates. d, e, Quantification of lung metastases 12 d after i.v. injection (d) or 27 d after excision of a primary tumour induced by s.c. injection (e) of B16 or LLC1 TCs into Casp8ECKO animals. Cre-negative littermates served as control. No significant differences in primary tumour growth were observed (data not shown). f–h, Effect of z-VAD-fmk (zVAD, 100 μM) on B16 TC proliferation (f), viability (g) and migration (h). i, Representative confocal images of lung sections taken 6 h and images of whole lungs taken 12 d after i.v. injection of B16 TCs into WT animals treated with DMSO (control) or zVAD shortly before and at 3 h after TC injection (plus at 6 h for the 12 d experiment); scale bar, 50 μm. Shown are representative data of two (b, c, e) or three (d, f–h) independent experiments with mean values ± s.d. (b–e, h) or ± s.e.m. (f, g) from n = 3 (b) or n = 5 (d) animals per group or from n = 11 (B16, control), n = 7 (B16, Casp8ECKO), n = 8 (LLC1, control) and n = = 6 (LLC1, Casp8ECKO) animals (e) or from biological sextuplicates (n = 6) (c, f, g) or triplicates (n = 3) (h). *P < 0.05; ***P < 0.001; n.s., not significant. Unpaired, two-tailed Student’s t-test (b, d–h) or one-way ANOVA and Bonferroni’s post hoc test (c). For gel source data see Supplementary Fig. 1.
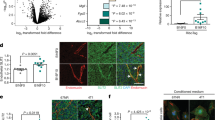
Extended Data Figure 7 DR6 is expressed in ECs, and DR6 expressed in immune cells is not involved in TC metastasis.
a, Western blot analysis of lysates from HUVECs transfected with scrambled siRNA (siCTRL) or different sets of siRNAs directed against mRNA encoding DR6 (siDR6). The antibody detects the 90 kDa glycosylated form of DR6. α-tubulin served as loading control. b, Expression levels of DR6 in different human or mouse ECs as determined by quantitative PCR. Shown are relative expression levels normalized to GAPDH levels. c, Confocal images of human tissues of the indicated origin stained for CD31 (red), DR6 (green) and cell nuclei (DAPI, blue) (left panel). Control-IgG antibody and donkey anti-rabbit secondary antibody coupled to AF488 served as negative controls (right panels); scale bar, 5 μm. d, Analysis of HUVEC single-cell gene expression for DR6 as determined by the 2LoDct-ct method and normalized to the cell with the highest expression level (100%). Each bar represents the gene expression level of one individual cell (data of a total of 80 cells analysed are shown). Single-cell analysis revealed that less than 10% of ECs express DR6. e–j, Irradiated DR6+/+ or DR6−/− animals were reconstituted with bone marrow cells from DR6+/+ or DR6−/− donor animals, respectively (DR6+/+ → DR6+/+, DR6−/− → DR6+/+ or DR6+/+ → DR6−/−) and quantitative PCR analysis of DR6 expression in PBMCs was performed (e) or bone marrow chimaeras as indicated were injected i.v. with B16 TCs and lungs were analysed after 6 h for pulmonary EthD-III-positive ECs (g) and extravascular TCs (h) or after 12 d for lung metastases (j). f, i, Representative confocal images of lung sections stained for the indicated markers (f) and images of whole lungs (i); scale bar, 50 μm. Shown are representative data of two independent experiments with mean values ± s.d. (e, j) or ± s.e.m. (g, h) from n = 3 (e) or n = 4 (g, h) animals per condition or n = 6 (DR6+/+ → DR6+/+), n = 4 (DR6−/− → DR6+/+) and n = 5 (DR6+/+ → DR6−/−) animals (j). **P < 0.01; n.s., not significant. One-way ANOVA and Bonferroni’s post hoc test. For gel source data see Supplementary Fig. 1.
Extended Data Figure 8 Effects of anti-DR6 antibody and DR6-Fc on tumour metastasis.
a–i, Quantification of MDA-MB-231 TC-induced EC necroptosis (a, c, g), transmigrated TCs over an endothelial layer (b, e, h), B16 TC-induced EC necroptosis after i.v. injection into C57BL/6 WT animals (d) or lung metastases 12 d after i.v. injection of LLC1 TCs into WT animals (f, i) upon treatment with an anti-DR6 antibody (5D10) (a, b, f), etanercept (c–e) or the extracellular domain of DR6 fused to DR6 (DR6-Fc) (g–j). Animals were treated shortly before and 3 h after TC injection (d) as well as 6 h after TC injection (f, i). PBS, an IgG1 antibody or the Fc domain of IgG1 or IgG2A (IgG1-Fc, IgG2A-Fc) served as controls. g, Representative confocal images of lung sections taken 6 h and images of whole lungs taken 12 d after i.v. injection of B16 TCs into WT animals treated with IgG2A-Fc (control) or DR6-Fc shortly before and at 3 h after TC injection (plus at 6 h for the 12 d experiment); scale bar, 50 μm. Shown are representative data of three independent experiments with mean values ± s.e.m. (a–e, g, h) or ± s.d. (f, i) from biological sextuplicates (n = 6) (a–c, e, g, h) or from n = 3 (d) or n = 4 (f, i) animals per group. *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant. Two-way ANOVA and Bonferroni’s post hoc test (a–d, g, h) or unpaired, two-tailed Student’s t-test (e, f, i).
Extended Data Figure 9 APP is expressed in different murine and human TCs.
a, Expression levels of APP in different human and mouse TCs as determined by quantitative PCR. Shown are relative expression levels normalized to GAPDH levels. b, MDA-MB-231 single-cell gene expression analysis for APP as determined by the 2LoDct-ct method and normalized to the cell with the highest expression level (100%). Each bar represents the gene expression level of one individual cell (data of a total of 78 cells analysed are shown). Single-cell analysis revealed that all TCs express APP. c, Analysis of knockdown efficiency in MDA-MB-231 TCs using different siRNAs against APP (siAPP). Shown is the relative mRNA expression normalized to GAPDH levels and to the level detected in scramble siRNA-treated samples (siCTRL). d, Quantification of EC necroptosis in HUVECs cultured in the presence of MDA-MB-231 TCs (direct contact) or exposed to the supernatant of TC–EC co-cultures after 18 h of culture (condit. medium). e, Western blot analysis of cell lysates or conditioned media (supernat.) of parental HEK293T (HEK) cells, HEK cells stably expressing membrane-bound full-length APP695 (HEKAPP), mock-transfected COS-1 cells (COS-1) or COS-1 cells transiently expressing membrane-bound full-length APP695 (COS-1APP) or soluble APPsα (COS-1APPsα). Note that secreted APPsα from COS-1 cells (supernat.) compared with APPsα found in the corresponding cell lysates is glycosylated and thus runs at higher molecular mass. Anti-APP (22C11) was used to detect APP. Membranes were cut to detect β-tubulin as loading control. Shown are representative data of three independent experiments with mean values ± s.e.m. from biological sextuplicates (n = 6). **P < 0.01; ***P < 0.001. Two-way ANOVA and Bonferroni’s post hoc test. For gel source data see Supplementary Fig. 1.
Extended Data Figure 10 Effects of loss of APP on TCs and on metastasis formation.
a, Analysis of knockdown efficiency in B16 and LLC1 TCs using different siRNAs against mRNA encoding APP (siAPP). Shown is the relative mRNA expression normalized to GAPDH levels and to the level detected in scramble siRNA-treated samples (siCTRL). b–d, Knockdown of APP in B16 or LLC1 TCs (B16siAPP and LLC1siAPP) and evaluation of cell proliferation (b), viability (c) and migration (d). e, f, Evaluation of APP-deficient TC-induced EC death in vitro in C57BL/6 WT primary MLECs (e) and the ability of APP-deficient TCs to migrate over an endothelial layer (f). g, Quantification of lung metastases 12 d after i.v. injection of LLC1 TCs with silenced APP expression (LLC1siAPP) into WT animals. Shown are representative data of three independent experiments with mean values ± s.e.m. (b, c, e) or ± s.d. (d, f, g) from biological sextuplicates (n = 6) (b, c, e, f), triplicates (n = 3) (d) or from n = 5 animals per condition (g). *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant. Unpaired, two-tailed Student’s t-test (b, c, f) or one-way ANOVA and Bonferroni’s post hoc test (e, g). h, Model: TCs induce RIPK1/RIPK3/MLKL-dependent necroptosis in ECs via APP-DR6. TCs then may directly pass through the emerging gap after EC death. Alternatively, or in parallel, damage-associated molecular pattern molecules (DAMPs) released from necroptotic ECs could act on TCs and/or non-necroptotic endothelial as well as other cells to promote TC extravasation and metastasis.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-2, original source images for data obtained by electrophoretic separation. (PDF 4521 kb)
Supplementary Table 1
This table contains information regarding the identification of endothelial receptors involved in the regulation of tumour cell-induced endothelial cell death (expression data, siRNA target sequences). (XLSX 24 kb)
Examples of endothelial cells undergoing necroptosis after contact with a tumour cell
Time lapse imaging of HUVEC upon contact with MDA-MB-231 tumour cells (GFP, green). Hoechst33342 labels all cell nuclei (blue) and EthD-III (red) labels necroptotic cells. Imaging shows endothelial cells that undergo necroptotic cell death (arrow head) several hours after contact with a tumour cell. (MP4 6260 kb)
Examples of endothelial cells undergoing necroptosis after contact with a tumour cell
Time lapse imaging of HUVEC upon contact with MDA-MB-231 tumour cells (GFP, green). Hoechst33342 labels all cell nuclei (blue) and EthD-III (red) labels necroptotic cells. Imaging shows endothelial cells that undergo necroptotic cell death (arrow head) several hours after contact with a tumour cell. (MP4 5806 kb)
Examples of endothelial cells undergoing necroptosis after contact with a tumour cell
Time lapse imaging of HUVEC upon contact with MDA-MB-231 tumour cells (GFP, green). Hoechst33342 labels all cell nuclei (blue) and EthD-III (red) labels necroptotic cells. Imaging shows endothelial cells that undergo necroptotic cell death (arrow head) several hours after contact with a tumour cell. (MP4 7158 kb)
Morphological criteria for the distinction between living, apoptotic and necrotic endothelial cells
Time lapse imaging of HUVEC cultured in the presence of PBS (control) or in the presence of H2O2 (1 mM) to induce necrosis or TNFα (100 ng/ml) to induce apoptosis. Cell nuclei were stained with Hoechst33342 (blue). Nuclei of necrotic cells stained positive for the membrane-impermeant nuclear dye EthD-III (red). Living cells appear with normal round to kidney-shaped nuclei and are negative for EthD-III. Necrotic cells appear with normal round to kidney-shaped nuclei or with minor degrees of nuclear shrinkage (no condensation and no fragmentation) and are positive for EthD-III. Apoptotic cells appear with strong condensed and frequently fragmented nuclei and are negative for EthD-III. No late apoptotic cells are shown in the videos. (MP4 2867 kb)
Rights and permissions
About this article
Cite this article
Strilic, B., Yang, L., Albarrán-Juárez, J. et al. Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis. Nature 536, 215–218 (2016). https://doi.org/10.1038/nature19076
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature19076
This article is cited by
-
Identification of TNFRSF21 as an inhibitory factor of osteosarcoma based on a necroptosis-related prognostic gene signature and molecular experiments
Cancer Cell International (2024)
-
Age-related noncanonical TRMT6–TRMT61A signaling impairs hematopoietic stem cells
Nature Aging (2024)
-
Necroptosis inhibitors: mechanisms of action and therapeutic potential
Apoptosis (2024)
-
Necroptosis-related LncRNAs in skin cutaneous melanoma: evaluating prognosis, predicting immunity, and guiding therapy
BMC Cancer (2023)
-
A tissue-engineered model of the blood-tumor barrier during metastatic breast cancer
Fluids and Barriers of the CNS (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.