Abstract
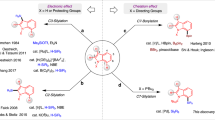
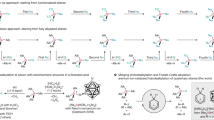
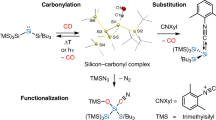
Heteroaromatic compounds containing carbon–silicon (C–Si) bonds are of great interest in the fields of organic electronics and photonics1, drug discovery2, nuclear medicine3 and complex molecule synthesis4,5,6, because these compounds have very useful physicochemical properties. Many of the methods now used to construct heteroaromatic C–Si bonds involve stoichiometric reactions between heteroaryl organometallic species and silicon electrophiles6,7 or direct, transition-metal-catalysed intermolecular carbon–hydrogen (C–H) silylation using rhodium or iridium complexes in the presence of excess hydrogen acceptors8,9. Both approaches are useful, but their limitations include functional group incompatibility, narrow scope of application, high cost and low availability of the catalysts, and unproven scalability. For this reason, a new and general catalytic approach to heteroaromatic C–Si bond construction that avoids such limitations is highly desirable. Here we report an example of cross-dehydrogenative heteroaromatic C–H functionalization catalysed by an Earth-abundant alkali metal species. We found that readily available and inexpensive potassium tert-butoxide catalyses the direct silylation of aromatic heterocycles with hydrosilanes, furnishing heteroarylsilanes in a single step. The silylation proceeds under mild conditions, in the absence of hydrogen acceptors, ligands or additives, and is scalable to greater than 100 grams under optionally solvent-free conditions. Substrate classes that are difficult to activate with precious metal catalysts are silylated in good yield and with excellent regioselectivity. The derived heteroarylsilane products readily engage in versatile transformations enabling new synthetic strategies for heteroaromatic elaboration, and are useful in their own right in pharmaceutical and materials science applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Zhang, F., Wu, D., Xu, Y. & Feng, X. Thiophene-based conjugated oligomers for organic solar cells. J. Mater. Chem. 21, 17590–17600 (2011)
Showell, G. A. & Mills, J. S. Chemistry challenges in lead optimization: silicon isosteres in drug discovery. Drug Discov. Today 8, 551–556 (2003)
Franz, A. K. & Wilson, S. O. Organosilicon molecules with medicinal applications. J. Med. Chem. 56, 388–405 (2013)
Ball, L. T., Lloyd-Jones, G. C. & Russell, C. A. Gold-catalyzed direct arylation. Science 337, 1644–1648 (2012)
Denmark, S. E. & Baird, J. D. Palladium-catalyzed cross-coupling reactions of silanolates: a paradigm shift in silicon-based cross-coupling reactions. Chem. Eur. J. 12, 4954–4963 (2006)
Langkopf, E. & Schinzer, D. Uses of silicon-containing compounds in the synthesis of natural products. Chem. Rev. 95, 1375–1408 (1995)
Whisler, M. C., MacNeil, S., Snieckus, V. & Beak, P. Beyond thermodynamic acidity: A perspective on the complex-induced proximity effect (CIPE) in deprotonation reactions. Angew. Chem. Int. Ed. 43, 2206–2225 (2004)
Cheng, C. & Hartwig, J. F. Rhodium-catalyzed intermolecular C–H silylation of arenes with high steric regiocontrol. Science 343, 853–857 (2014)
Lu, B. & Falck, J. R. Efficient iridium-catalyzed C–H functionalization/silylation of heteroarenes. Angew. Chem. Int. Ed. 47, 7508–7510 (2008)
Tamao, K., Uchida, M., Izumizawa, T., Furukawa, K. & Yamaguchi, S. Silole derivatives as efficient electron transporting materials. J. Am. Chem. Soc. 118, 11974–11975 (1996)
Ting, R., Adam, M. J., Ruth, T. J. & Perrin, D. M. Arylfluoroborates and alkylfluorosilicates as potential PET imaging agents: high-yielding aqueous biomolecular 18F-labeling. J. Am. Chem. Soc. 127, 13094–13095 (2005)
Du, W., Kaskar, B., Blumbergs, P., Subramanian, P. -K. & Curran, D. P. Semisynthesis of DB-67 and other silatecans from camptothecin by thiol-promoted addition of silyl radicals. Bioorg. Med. Chem. 11, 451–458 (2003)
Furukawa, S., Kobayashi, J. & Kawashima, T. Development of a sila-Friedel–Crafts reaction and its application to the synthesis of dibenzosilole derivatives. J. Am. Chem. Soc. 131, 14192–14193 (2009)
Curless, L. D., Clark, E. R., Dunsford, J. J. & Ingleson, M. J. E–H (E = R3Si or H) bond activation by B(C6F5)3 and heteroarenes; competitive dehydrosilylation, hydrosilylation and hydrogenation. Chem. Commun. 50, 5270–5272 (2014)
Klare, H. F. T. et al. Cooperative catalytic activation of Si–H bonds by a polar Ru–S bond: regioselective low-temperature C–H silylation of indoles under neutral conditions by a Friedel-Crafts mechanism. J. Am. Chem. Soc. 133, 3312–3315 (2011)
Seregin, I. V. & Gevorgyan, V. Direct transition metal-catalyzed functionalization of heteroaromatic compounds. Chem. Soc. Rev. 36, 1173–1193 (2007)
Fedorov, A., Toutov, A. A., Swisher, N. A. & Grubbs, R. H. Lewis-base silane activation: from reductive cleavage of aryl ethers to selective ortho-silylation. Chem. Sci. 4, 1640–1645 (2013)
Weickgenannt, A. & Oestreich, M. Potassium tert-butoxide-catalyzed dehydrogenative Si–O coupling: reactivity pattern and mechanism of an underappreciated alcohol protection. Chem. Asian J. 4, 406–410 (2009)
Song, J. J. et al. Organometallic methods for the synthesis and functionalization of azaindoles. Chem. Soc. Rev. 36, 1120–1132 (2007)
Li, C.-J. & Trost, B. M. Green chemistry for chemical synthesis. Proc. Natl Acad. Sci. USA 105, 13197–13202 (2008)
Collins, K. D. & Glorius, F. A robustness screen for the rapid assessment of chemical reactions. Nature Chem. 5, 597–601 (2013)
Seiple, I. B. et al. Direct C−H arylation of electron-deficient heterocycles with arylboronic acids. J. Am. Chem. Soc. 132, 13194–13196 (2010)
Zhao, Z. & Snieckus, V. Directed ortho metalation-based methodology. Halo-, nitroso-, and boro-induced ipso-desilylation. Link to an in situ Suzuki reaction. Org. Lett. 7, 2523–2526 (2005)
Lee, M., Ko, S. & Chang, S. Highly selective and practical hydrolytic oxidation of organosilanes to silanols catalyzed by a ruthenium complex. J. Am. Chem. Soc. 122, 12011–12012 (2000)
Hansen, M. M. et al. Lithiated benzothiophenes and benzofurans require 2-silyl protection to avoid anion migration. Synlett 8, 1351–1354 (2004)
Wang, Y. & Watson, M. D. Transition-metal-free synthesis of alternating thiophene-perfluoroarene copolymers. J. Am. Chem. Soc. 128, 2536–2537 (2006)
Kuznetsov, A., Onishi, Y., Inamoto, Y. & Gevorgyan, Y. Fused heteroaromatic dihydrosiloles: synthesis and double-fold modification. Org. Lett. 15, 2498–2501 (2013)
Oyamada, J., Nishiura, M. & Hou, Z. Scandium-catalyzed silylation of aromatic C–H bonds. Angew. Chem. Int. Ed. 50, 10720–10723 (2011)
Kakiuchi, F., Tsuchiya, K., Matsumoto, M., Mizushima, E. & Chatani, N. Ru3(CO)12-catalyzed silylation of benzylic C–H bonds in arylpyridines and arylpyrazoles with hydrosilanes via C-H bond cleavage. J. Am. Chem. Soc. 126, 12792–12793 (2004)
Sakakura, T., Tokunaga, Y., Sodeyama, T. & Tanaka, M. Catalytic C–H activation. Silylation of arenes with hydrosilane or disilane by RhCl(CO)(PMe3)2 under irradiation. Chem. Lett. 16, 2375–2378 (1987)
Acknowledgements
This work was supported by the NSF under the CCI Center for Selective C–H Functionalization (CHE-1205646) and under CHE-1212767, and by BP under the XC2 initiative. We thank the Novartis Institutes for Biomedical Research Incorporated for the donation of samples to the CCHF. D. Morton is thanked for a donation of thenalidine. A.A.T. is grateful to the Resnick Sustainability Institute at Caltech and to Dow Chemical for a predoctoral fellowship, and to NSERC for a PGS D fellowship. The Shanghai Institute of Organic Chemistry (SIOC) and S.-L. You are thanked for a postdoctoral fellowship to W.-B.L. We thank S. Virgil and the Caltech Center for Catalysis and Chemical Synthesis for access to analytical equipment. D. Vandervelde is acknowledged for assistance with NMR interpretation. N. Dalleska is thanked for assistance with ICP-MS trace metal analysis. M. Shahgoli and N. Torian are acknowledged for assistance with high-resolution mass spectrometry.
Author information
Authors and Affiliations
Contributions
A.A.T., W.-B.L. and K.N.B. developed the reactions, performed the experiments and analysed data. A.F. analysed data. A.A.T and R.H.G. had the idea for and directed the investigations with W.-B.L. and B.M.S. A.A.T. and W.-B.L. prepared the manuscript with contributions from all authors. All authors contributed to discussions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data – see Contents for details. (PDF 7491 kb)
Rights and permissions
About this article
Cite this article
Toutov, A., Liu, WB., Betz, K. et al. Silylation of C–H bonds in aromatic heterocycles by an Earth-abundant metal catalyst. Nature 518, 80–84 (2015). https://doi.org/10.1038/nature14126
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14126
This article is cited by
-
Intermolecular trans-bis-silylation of terminal alkynes
Nature Synthesis (2023)
-
Stepwise on-demand functionalization of multihydrosilanes enabled by a hydrogen-atom-transfer photocatalyst based on eosin Y
Nature Chemistry (2023)
-
Catalytic asymmetric silicon-carbon bond-forming transformations based on Si-H functionalization
Science China Chemistry (2023)
-
Rhodium hydride enabled enantioselective intermolecular C–H silylation to access acyclic stereogenic Si–H
Nature Communications (2022)
-
Phosphorus(III)-assisted regioselective C–H silylation of heteroarenes
Nature Communications (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.