Abstract
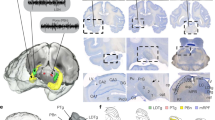
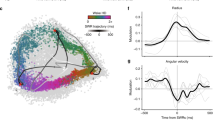
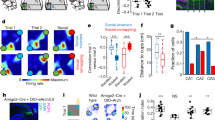
Hippocampal ripples, episodic high-frequency field-potential oscillations primarily occurring during sleep and calmness, have been described in mice, rats, rabbits, monkeys and humans, and so far they have been associated with retention of previously acquired awake experience. Although hippocampal ripples have been studied in detail using neurophysiological methods, the global effects of ripples on the entire brain remain elusive, primarily owing to a lack of methodologies permitting concurrent hippocampal recordings and whole-brain activity mapping. By combining electrophysiological recordings in hippocampus with ripple-triggered functional magnetic resonance imaging, here we show that most of the cerebral cortex is selectively activated during the ripples, whereas most diencephalic, midbrain and brainstem regions are strongly and consistently inhibited. Analysis of regional temporal response patterns indicates that thalamic activity suppression precedes the hippocampal population burst, which itself is temporally bounded by massive activations of association and primary cortical areas. These findings suggest that during off-line memory consolidation, synergistic thalamocortical activity may be orchestrating a privileged interaction state between hippocampus and cortex by silencing the output of subcortical centres involved in sensory processing or potentially mediating procedural learning. Such a mechanism would cause minimal interference, enabling consolidation of hippocampus-dependent memory.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Squire, L. R. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 99, 195–231 (1992)
Eichenbaum, H. Declarative memory: insights from cognitive neurobiology. Annu. Rev. Psychol. 48, 547–572 (1997)
Nadel, L. & Hardt, O. Update on memory systems and processes. Neuropsychopharmacology 36, 251–273 (2011)
Buzsáki, G. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience 31, 551–570 (1989)
Buzsáki, G. The hippocampo–neocortical dialogue. Cereb. Cortex 6, 81–92 (1996)
Hasselmo, M. E. Neuromodulation and the hippocampus: memory function and dysfunction in a network simulation. Prog. Brain Res. 121, 3–18 (1999)
Pennartz, C. M. A., Uylings, H. B. M., Barnes, C. A. & McNaughton, B. L. Memory reactivation and consolidation during sleep: from cellular mechanisms to human performance. Prog. Brain. Res. 138, 143–166 (2002)
Eichenbaum, H. A cortical-hippocampal system for declarative memory. Nature Rev. Neurosci. 1, 41–50 (2000)
Buzsáki, G., Horvath, Z., Urioste, R., Hetke, J. & Wise, K. High-frequency network oscillation in the hippocampus. Science 256, 1025–1027 (1992)
O'Keefe, J. & Nadel, L. The Hippocampus as a Cognitive Map 114–152 (Oxford Univ. Press, 1978)
Skaggs, W. E. et al. EEG sharp waves and sparse ensemble unit activity in the macaque hippocampus. J. Neurophysiol. 98, 898–910 (2007)
Axmacher, N., Elger, C. E. & Fell, J. Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain 131, 1806–1817 (2008)
Ylinen, A. et al. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J. Neurosci. 15, 30–46 (1995)
Girardeau, G. & Zugaro, M. Hippocampal ripples and memory consolidation. Curr. Opin. Neurobiol. 21, 452–459 (2011)
Wilson, M. A. & McNaughton, B. L. Reactivation of hippocampal ensemble memories during sleep. Science 265, 676–679 (1994)
Kudrimoti, H. S., Barnes, C. A. & McNaughton, B. L. Reactivation of hippocampal cell assemblies: effects of behavioral state, experience, and EEG dynamics. J. Neurosci. 19, 4090–4101 (1999)
Nádasdy, Z., Hirase, H., Czurko, A., Csicsvari, J. & Buzsaki, G. Replay and time compression of recurring spike sequences in the hippocampus. J. Neurosci. 19, 9497–9507 (1999)
Skaggs, W. E. & McNaughton, B. L. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science 271, 1870–1873 (1996)
Eschenko, O., Ramadan, W., Molle, M., Born, J. & Sara, S. J. Sustained increase in hippocampal sharp-wave ripple activity during slow-wave sleep after learning. Learn. Mem. 15, 222–228 (2008)
O'Neill, J., Senior, T. J., Allen, K., Huxter, J. R. & Csicsvari, J. Reactivation of experience-dependent cell assembly patterns in the hippocampus. Nature Neurosci. 11, 209–215 (2008)
Ramadan, W., Eschenko, O. & Sara, S. J. Hippocampal sharp wave/ripples during sleep for consolidation of associative memory. PloS ONE 4, e6697 (2009)
Girardeau, G., Benchenane, K., Wiener, S. I., Buzsaki, G. & Zugaro, M. B. Selective suppression of hippocampal ripples impairs spatial memory. Nature Neurosci. 12, 1222–1223 (2009)
Ego-Stengel, V. & Wilson, M. A. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus 20, 1–10 (2010)
Siapas, A. G. & Wilson, M. A. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron 21, 1123–1128 (1998)
Wierzynski, C. M., Lubenov, E. V., Gu, M. & Siapas, A. G. State-dependent spike-timing relationships between hippocampal and prefrontal circuits during sleep. Neuron 61, 587–596 (2009)
Isomura, Y. et al. Integration and segregation of activity in entorhinal–hippocampal subregions by neocortical slow oscillations. Neuron 52, 871–882 (2006)
Sirota, A., Csicsvari, J., Buhl, D. & Buzsaki, G. Communication between neocortex and hippocampus during sleep in rodents. Proc. Natl Acad. Sci. USA 100, 2065–2069 (2003)
Steriade, M., Nunez, A. & Amzica, F. A novel slow ( 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J. Neurosci. 13, 3252–3265 (1993)
McCormick, D. A. & Bal, T. Sleep and arousal—thalamocortical mechanisms. Annu. Rev. Neurosci. 20, 185–215 (1997)
Mölle, M., Marshall, L., Gais, S. & Born, J. Grouping of spindle activity during slow oscillations in human non-rapid eye movement sleep. J. Neurosci. 22, 10941–10947 (2002)
Amzica, F. & Steriade, M. The K-complex: its slow ( 1-Hz) rhythmicity and relation to delta waves. Neurology 49, 952–959 (1997)
Battaglia, F. P., Sutherland, G. R. & McNaughton, B. L. Hippocampal sharp wave bursts coincide with neocortical “up-state” transitions. Learn. Mem. 11, 697–704 (2004)
Mölle, M., Yeshenko, O., Marshall, L., Sara, S. J. & Born, J. Hippocampal sharp wave-ripples linked to slow oscillations in rat slow-wave sleep. J. Neurophysiol. 96, 62–70 (2006)
Clemens, Z. et al. Temporal coupling of parahippocampal ripples, sleep spindles and slow oscillations in humans. Brain 130, 2868–2878 (2007)
Axmacher, N., Mormann, F., Fernandez, G., Elger, C. E. & Fell, J. Memory formation by neuronal synchronization. Brain Res. Rev. 52, 170–182 (2006)
Born, J. Slow-wave sleep and the consolidation of long-term memory. World J. Biol. Psychiatry 11, 16–21 (2010)
Steriade, M. The corticothalamic system in sleep. Front. Biosci. 8, d878–d899 (2003)
Sullivan, D. et al. Relationships between hippocampal sharp waves, ripples, and fast gamma oscillation: influence of dentate and entorhinal cortical activity. J. Neurosci. 31, 8605–8616 (2011)
Paxinos, G., Huang, X. F., Petrides, M. & Toga, A. W. The Rhesus Monkey Brain in Stereotactic Coordinates (Elsevier, 2008)
Shmuel, A., Augath, M. A., Oeltermann, A. & Logothetis, N. K. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nature Neurosci. 9, 569–577 (2006)
Logothetis, N. K. et al. The effects of electrical microstimulation on cortical signal propagation. Nature Neurosci. 13, 1283–1291 (2010)
Vincent, J. L. et al. Coherent spontaneous activity identifies a hippocampal–parietal mnemonic network. J. Neurophysiol. 96, 3517–3531 (2006)
Poldrack, R. A. & Packard, M. G. Competition among multiple memory systems: converging evidence from animal and human brain studies. Neuropsychologia 41, 245–251 (2003)
Schroeder, J. P., Wingard, J. C. & Packard, M. G. Post-training reversible inactivation of hippocampus reveals interference between memory systems. Hippocampus 12, 280–284 (2002)
Oliveira, A. M., Hawk, J. D., Abel, T. & Havekes, R. Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Learn. Mem. 17, 155–160 (2010)
Poldrack, R. A. & Rodriguez, P. How do memory systems interact? Evidence from human classification learning. Neurobiol. Learn. Mem. 82, 324–332 (2004)
Mouret, J., Jeannero, M. & Jouvet, M. L′activité électrique du systeme visuel au cours de la phase paradoxale du sommeil chez le chat. J. Physiol. (Paris). 55, 305–306 (1963)
Wolansky, T., Clement, E. A., Peters, S. R., Palczak, M. A. & Dickson, C. T. Hippocampal slow oscillation: a novel EEG state and its coordination with ongoing neocortical activity. J. Neurosci. 26, 6213–6229 (2006)
Axmacher, N., Elger, C. E. & Fell, J. Memory formation by refinement of neural representations: the inhibition hypothesis. Behav. Brain Res. 189, 1–8 (2008)
Logothetis, N. K. What we can do and what we cannot do with fMRI. Nature 453, 869–878 (2008)
Acknowledgements
We thank D. Omer and M. Munk for reading the manuscript and for useful suggestions, D. Blaurock for English language corrections and editing, and P. Douay for help with the alert monkey experiments. This research was supported by the Max Planck Society. We apologize to those whose work we have not been able to cite for reasons of space.
Author information
Authors and Affiliations
Contributions
N.K.L and O.E. designed the experiments and carried out research. N.K.L. analysed the data, wrote the manuscript and supervised the research. Y.M. carried out research and, together with M.B., contributed data analysis. M.A. and T.S. collected the physiology fMRI data, H.C.E. helped with all anatomical details required to define ROIs and enable three-dimensional registration of functional images to standard anatomical scans, and A.O. designed and developed all electronics and electrodes permitting concurrent multiple-contact electrophysiological recordings and fMRI.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Methods and References, Supplementary Figures 1-13, Supplementary Text and additional references. (PDF 6878 kb)
Rights and permissions
About this article
Cite this article
Logothetis, N., Eschenko, O., Murayama, Y. et al. Hippocampal–cortical interaction during periods of subcortical silence. Nature 491, 547–553 (2012). https://doi.org/10.1038/nature11618
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11618
This article is cited by
-
Awake ripples enhance emotional memory encoding in the human brain
Nature Communications (2024)
-
The generative grammar of the brain: a critique of internally generated representations
Nature Reviews Neuroscience (2024)
-
Augmenting hippocampal–prefrontal neuronal synchrony during sleep enhances memory consolidation in humans
Nature Neuroscience (2023)
-
How coupled slow oscillations, spindles and ripples coordinate neuronal processing and communication during human sleep
Nature Neuroscience (2023)
-
Closed-loop brain stimulation augments fear extinction in male rats
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.