Abstract
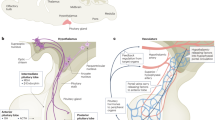
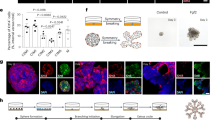
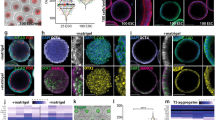
The adenohypophysis (anterior pituitary) is a major centre for systemic hormones. At present, no efficient stem-cell culture for its generation is available, partly because of insufficient knowledge about how the pituitary primordium (Rathke’s pouch) is induced in the embryonic head ectoderm. Here we report efficient self-formation of three-dimensional adenohypophysis tissues in an aggregate culture of mouse embryonic stem (ES) cells. ES cells were stimulated to differentiate into non-neural head ectoderm and hypothalamic neuroectoderm in adjacent layers within the aggregate, and treated with hedgehog signalling. Self-organization of Rathke’s-pouch-like three-dimensional structures occurred at the interface of these two epithelia, as seen in vivo, and various endocrine cells including corticotrophs and somatotrophs were subsequently produced. The corticotrophs efficiently secreted adrenocorticotropic hormone in response to corticotrophin releasing hormone and, when grafted in vivo, these cells rescued the systemic glucocorticoid level in hypopituitary mice. Thus, functional anterior pituitary tissue self-forms in ES cell culture, recapitulating local tissue interactions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kelberman, D., Rizzoti, K., Lovell-Badge, R., Robinson, I. C. & Dattani, M. T. Genetic regulation of pituitary gland development in human and mouse. Endocr. Rev. 30, 790–829 (2009)
Romero, C. J., Nesi-França, S. & Radovick, S. The molecular basis of hypopituitarism. Trends Endocrinol. Metab. 20, 506–516 (2009)
Rizzoti, K. & Lovell-Badge, R. Early development of the pituitary gland: induction and shaping of Rathke’s pouch. Rev. Endocr. Metab. Disord. 6, 161–172 (2005)
Takuma, N. et al. Formation of Rathke’s pouch requires dual induction from the diencephalon. Development 125, 4835–4840 (1998)
Suh, H., Gage, P. J., Drouin, J. & Camper, S. A. Pitx2 is required at multiple stages of pituitary organogenesis: pituitary primordium formation and cell specification. Development 129, 329–337 (2002)
Zhu, X., Gleiberman, A. S. & Rosenfeld, M. G. Molecular physiology of pituitary development: signaling and transcriptional networks. Physiol. Rev. 87, 933–963 (2007)
Rizzoti, K. & Lovell-Badge, R. Early development of the pituitary gland: induction and shaping of Rathke’s pouch. Rev. Endocr. Metab. Disord. 6, 161–172 (2005)
Ericson, J., Norlin, S., Jessell, T. M. & Edlund, T. Integrated FGF and BMP signaling controls the progression of progenitor cell differentiation and the emergence of pattern in the embryonic anterior pituitary. Development 125, 1005–1015 (1998)
Wataya, T. et al. Minimization of exogenous signals in ES cell culture induces rostral hypothalamic differentiation. Proc. Natl Acad. Sci. USA 105, 11796–11801 (2008)
Watanabe, K. et al. Directed differentiation of telencephalic precursors from embryonic stem cells. Nature Neurosci. 8, 288–296 (2005)
Eiraku, M. et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3, 519–532 (2008)
Basch, M. L. & Bronner-Fraser, M. Neural crest inducing signals. Adv. Exp. Med. Biol. 589, 24–31 (2006)
Eiraku, E. et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51–56 (2011)
Jones, C. M., Lyons, K. M. & Hogan, B. L. Involvement of bone morphogenetic protein-4 (BMP-4) and Vgr-1 in morphogenesis and neurogenesis in the mouse. Development 111, 531–542 (1991)
Danjo, T. et al. Subregional specification of ES cell-derived ventral telencephalic tissues by timed and combinatory treatment with extrinsic signals. J. Neurosci. 31, 1919–1933 (2011)
Treier, M. et al. Hedgehog signaling is required for pituitary gland development. Development 128, 377–386 (2001)
Ellsworth, B. S., Butts, D. L. & Camper, S. A. Mechanisms underlying pituitary hypoplasia and failed cell specification in Lhx3-deficient mice. Dev. Biol. 313, 118–129 (2008)
Sheng, H. Z. et al. Specification of pituitary cell lineages by the LIM homeobox gene Lhx3. Science 272, 1004–1007 (1996)
Kikuchi, M. et al. Changes in E- and N-cadherin expression in developing rat adenohypophysis. Anat. Rec. 290, 486–490 (2007)
Thor, S., Ericson, J., Brännström, T. & Edlund, T. The homeodomain LIM protein Isl-1 is expressed in subsets of neurons and endocrine cells in the adult rat. Neuron 7, 881–889 (1991)
Bharti, K., Gasper, M., Bertuzzi, S. & Arnheiter, H. Lack of the ventral anterior homeodomain transcription factor VAX1 leads to induction of a second pituitary. Development 138, 873–878 (2011)
Mason, I. Initiation to end point: the multiple roles of fibroblast growth factors in neural development. Nature Rev. Neurosci. 8, 583–596 (2007)
Davis, S. W., Mortensen, A. H. & Camper, S. A. Birthdating studies reshape models for pituitary gland cell specification. Dev. Biol. 352, 215–227 (2011)
Lamolet, B. et al. A pituitary cell-restricted T box factor, Tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell 104, 849–859 (2001)
Zhu, X. et al. Sustained Notch signaling in progenitors is required for sequential emergence of distinct cell lineages during organogenesis. Genes Dev. 20, 2739–2753 (2006)
Kita, A. et al. Hes1 and Hes5 control the progenitor pool, intermediate lobe specification, and posterior lobe formation in the pituitary development. Mol. Endocrinol. 21, 1458–1466 (2007)
Zhao, Y. et al. Reduced expression of the LIM-homeobox gene Lhx3 impairs growth and differentiation of Rathke’s pouch and increases cell apoptosis during mouse pituitary development. Mech. Dev. 123, 605–613 (2006)
Davis, S. W. et al. Molecular mechanisms of pituitary organogenesis: in search of novel regulatory genes. Mol. Cell. Endocrinol. 323, 4–19 (2010)
Hemming, F. J., Bégeot, M., Dubois, M. P. & Dubois, P. M. Fetal rat somatotropes in vitro: effects of insulin, cortisol, and growth hormone-releasing factor on their differentiation: a light and electron microscopic study. Endocrinology 114, 2107–2113 (1984)
Ogasawara, K. et al. Hormonal regulation of prolactin cell development in the fetal pituitary gland of the mouse. Endocrinology 150, 1061–1068 (2009)
Gleiberman, A. S., Fedtsova, N. G. & Rosenfeld, M. G. Tissue interactions in the induction of anterior pituitary: role of the ventral diencephalon, mesenchyme, and notochord. Dev. Biol. 213, 340–353 (1999)
Ingle, D. J. The effects of administering large amounts of cortin on the adrenal cortices of normal and hypophysectomized rats. Am. J. Physiol. 124, 369–371 (1938)
Falconi, G. & Rossi, G. L. Transauricular hypophysectomy in rats and mice. Endocrinology 74, 301–303 (1964)
Melmed, S. The Pituitary 3rd edn, 61 (Academic, 2011)
Acknowledgements
We are grateful to H. Enomoto, R. Ladher and M. Eiraku for invaluable comments, to K. Misaki for electron microscopy analysis, and to members of the Y.S. laboratory for discussion. This work was supported by grants-in-aid from Ministry of Education, Culture, Sports, Science and Technology (Y.S., Y.O.), the Knowledge Cluster Initiative at Kobe, and the Leading Project for Realization of Regenerative Medicine (Y.S.).
Author information
Authors and Affiliations
Contributions
H.S. and Y.S. designed the project and wrote the manuscript. H.S., T.K., M.O. and M.M. performed the experiments with the technical help and advice of T.N., N.T., M.S., K.M., H.M., S.Y. and T.W., and Y.O. provided critical advice on the research strategy and design.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-9 with legends and legends for Supplementary Movies 1-2. (PDF 13724 kb)
Supplementary Movie 1
This movie shows the formation of Lim3+ vesicles in SFEBq-cultured ESC Aggregates - see Supplementary Information file for full legend. (MOV 15058 kb)
Supplementary Movie 2
This movie shows improved locomotor activity in hypophysectomized mice receiving SAG+DAPT-treated aggregates - see Supplementary Information file for full legend. (MOV 13716 kb)
Rights and permissions
About this article
Cite this article
Suga, H., Kadoshima, T., Minaguchi, M. et al. Self-formation of functional adenohypophysis in three-dimensional culture. Nature 480, 57–62 (2011). https://doi.org/10.1038/nature10637
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10637
This article is cited by
-
Pituitary stem cells: past, present and future perspectives
Nature Reviews Endocrinology (2024)
-
Human 3D brain organoids: steering the demolecularization of brain and neurological diseases
Cell Death Discovery (2023)
-
Human-specific genetics: new tools to explore the molecular and cellular basis of human evolution
Nature Reviews Genetics (2023)
-
Missing pieces of the pituitary puzzle: participation of extra-adenohypophyseal placode-lineage cells in the adult pituitary gland
Cell and Tissue Research (2023)
-
Self-organization, quality control, and preclinical studies of human iPSC-derived retinal sheets for tissue-transplantation therapy
Communications Biology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.