Abstract
We report on four cases of endocervical adenocarcinoma associated with lobular endocervical glandular hyperplasia using histochemical and immunohistochemical analyses. The patients ranged in age from 59 to 67 years (mean 62 years). Chief complaints were watery vaginal discharge in two cases, genital bleeding in one and no subjective symptoms in one. Cytological examinations of the cervical smears revealed adenocarcinoma cells and benign-looking glandular cells with intracytoplasmic golden-yellow mucin in all cases. Radical hysterectomy was performed in three patients, and simple total hysterectomy was performed in one. From surgical specimens, three tumors were diagnosed as mucinous adenocarcinoma and one was adenocarcinoma in situ. All adenocarcinomas were located proximally on the cervix, and did not involve the transformation zone. Adjacent to carcinoma tissues in the cervix, lobular endocervical glandular hyperplasia was detected. The cells of lobular endocervical glandular hyperplasia were dominantly positive with neutral mucin, and immunohistochemistry revealed that these cells had prominent pyloric gland mucin (HIK1083). Focal immunopositivity for pyloric mucin was also observed in three adenocarcinomas. Either CEA or p53 were immunopositive in all adenocarcinomas and negative in the tissues of lobular endocervical glandular hyperplasia. Histopathological features of the present cases suggest that some endocervical adenocarcinomas may originate from lobular endocervical glandular hyperplasia.
Similar content being viewed by others
Main
Endocervical adenocarcinomas account for 10–15% of all cervical cancers and have been increasing in relative and absolute numbers.1, 2, 3, 4 Therefore, understanding the precursor lesions of endocervical adenocarcinoma has become more important for gynecologists and pathologists. Endocervical glandular dysplasia and atypical tubal metaplasia are recognized premalignant lesions, and a considerable number of studies on these lesions have been conducted so far.5, 6, 7, 8, 9, 10, 11, 12, 13 Consequently, the question arises whether other precursors exist. Microglandular hyperplasia,14, 15, 16, 17 mesonephric hyperplasia,18, 19 tunnel clusters,20, 21, 22 diffuse laminar endocervical glandular hyperplasia23 and lobular endocervical glandular hyperplasia are currently considered benign hyperplastic glandular lesions in the uterine cervix,24 but little is really known about their relationship with endocervical carcinomas.25, 26 Recently, several studies have focused on gastric mucin (or pyloric gland mucin) in glandular lesions of the cervix and its connection with endocervical glands' carcinogenesis.
Gastric phenotype has been described in endocervical gastric metaplasia, lobular endocervical glandular hyperplasia and minimal deviation adenocarcinomas from the findings of histochemistry and immunohistochemistry using monoclonal antibody (HIK1083) against pyloric gland mucin.27, 28, 29, 30, 31, 32, 33, 34, 35, 36 There is also speculation that hyperplastic or metaplastic glandular lesions with gastric mucin are precursors of endocervical adenocarcinoma or in situ lesions of minimal deviation adenocarcinoma when the correspondent mucin phenotype of these lesions is considered.32, 34, 37 However, this is still under discussion and, to our knowledge, there has been no direct evidence to support this hypothesis. In the current study, we report on four cases of endocervical adenocarcinoma associated with lobular endocervical glandular hyperplasia, and discuss the histogenesis of adenocarcinoma with respect to the presence of lobular endocervical glandular hyperplasia.
Materials and methods
Case Selection
Based on the criteria for the diagnosis of lobular endocervical glandular hyperplasia first proposed by Nucci et al,24 cases of endocervical neoplasms and endocervical proliferative disorders were re-evaluated by two pathologists (TK, SM) and one gynecologist (AH) in the surgical pathology files from 1996 to 2003 at University of Yamanashi Hospital, Yamanashi Central Hospital and Kofu Municipal Hospital. Subsequently, we retrieved 20 cases of lobular endocervical glandular hyperplasia and four cases of lobular endocervical glandular hyperplasia with adenocarcinoma. Hematoxylin and eosin-stained sections of the four cases of lobular endocervical glandular hyperplasia with adenocarcinoma were assessed for morphological features, and histochemistry and immunohistochemistry were performed on representative sections to characterize these lesions. Histological diagnosis of endocervical adenocarcinomas was through the established morphological classification of the World Health Organization, and clinical stages were evaluated by UICC/TNM classification.38, 39 To confirm cytological findings, Pap smears of the four cases were re-examined. Macroscopic findings were assessed through both macroscopic photographs of the surgical specimens and original pathological reports. Clinical details and follow-up information were obtained by communication with the original pathologists or the patients' physicians.
Histochemistry
Formalin-fixed and paraffin-embedded sections were cut at 3 μm and subjected to alcian blue/periodic acid-Schiff stain to demonstrate acidic mucin (blue) and neutral mucin (red) by light microscopy. The precise method has been described previously.40
Immunohistochemistry
Immunohistochemical stains were performed using the Ventana NX automated immunohistochemistry system (Ventana Medical Systems, Tucson, AZ, USA) with monoclonal/polyclonal primary antibodies to CEA (COL-1, Nichirei, Japan, dilution 1:1), p53 (DO-7, Novocastra, UK, dilution 1:100) and HIK1083 (Kanto kagaku, Japan, dilution 1:20). Formalin-fixed and paraffin-embedded sections were cut at 3 μm. Antigen retrieval was achieved by autoclave (110°C, 5 min) for p53, and microwave (1 min) for CEA. Sections processed without primary antibodies were employed as negative controls. Immunopositivities were assessed by light microscopy.
Results
Clinical Findings
Clinical data of patients are presented in Table 1 . All the patients were postmenopausal women, and the mean age was 62 years (range 59–67 years). Chief complaints were watery vaginal discharge in two patients (cases 1 and 2) and genital bleeding in one patient (case 4). One patient (case 3) consulted physicians because of an abnormal Pap smear during a health check. None of the cases had a history of hormonal therapy or usage of contraceptives. Radical hysterectomy was performed in three cases, and simple total hysterectomy was done in one case. One patient (case 3) received chemotherapy with cyclophosphamide, adriamycin and cysplatin after surgical excision. Case 4 received both chemotherapy and radiation therapy (45 Gy). No additional therapy was performed in cases 1 and 2. The follow-up period of this study ranged from 11 to 41 months, at which time three patients were alive without local recurrence or distant metastasis. One patient (case 4) died due to lung metastasis 13 months after surgery.
Macroscopic Findings
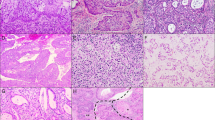
No apparent tumoral mass was seen on the surface of the uterine cervix (Figure 1a). In cut surfaces of the cervix, we detected lesions composed of aggregations of variable sized cysts (Figure 1b, c). These cystic lesions were from the proximal area of the cervix. Solid tumor adjacent to microcystic lesions was macroscopically identified only in case 4 (Figure 1d). The sizes of the lesions in all cases ranged from 14 to 19 mm in width and 8 to 14 mm in depth.
Gross appearance. (a) Non-fixed surgical specimen of the uterus (case 1). No apparent tumoral mass is detected on the surface of the uterine cervix. (b, case 1) (c, case 3) Cross-sections of fixed uterine cervixes. Aggregations of variable sized cysts are found in cut surface of the cervix (arrows). Arrowhead (c) designated the location where adenocarcinoma exists, which is confirmed by the histological examination, but macroscopically it is hard to recognize its presence. (d) Cross-section of the uterus (case 4). The lesions compose of solid tumor (arrowheads) with unclear border and microcystic lesions (arrows) are designated in the proximal of the cervix.
Microscopic Findings
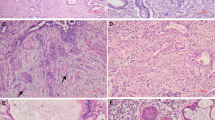
The lesions macroscopically recognized to be cystic were composed of dilated glands lined with high columnar mucinous cells. These columnar cells were frequently arranged in a lobular pattern without desmoplastic stromal reactions, and their nuclei were basal and uniform. These hyperplastic glandular lesions, diagnosed as lobular endocervical glandular hyperplasia (Figure 2a–d), occasionally had intranuclear cytoplasmic inclusions. Cancerous tissues were identified as minor components within hyperplastic lesions or adjacent to them. Carcinomas (Figure 3a–f) were diagnosed as invasive mucinous adenocarcinoma of endocervical type in cases 2, 3, and 4 (pTIa1 in cases 2 and 3, and pTIIIb in case 4), and case 1 was adenocarcinoma in situ (endocervical type) (pT0). No mitotic figures were noted in the tissues of lobular endocervical glandular hyperplasia, while they were frequently observed in adenocarcinomas. Both the adenocarcinomas and the lobular endocervical glandular hyperplasia tissue sections were from the proximal region of the cervix, and the adenocarcinoma tissues did not involve the transformation zone of the uterine cervix. No neoplastic squamous lesions were detected in the endocervix of any cases.
Histological findings of lobular endocervical glandular hyperplasia. (a, case 3) (c, case 2) Hyperplastic glandular lesions appear lobular arrangements without desmoplastic stromal reactions. (b, case 3) (d, case 2) Hyperplastic lesions compose of high columnar mucinous cells with no cellular atypia and lack of mitotic figure.
Histological findings of endocervical adenocarcinoma. (a, b) Adenocarcinoma in situ (case 1). Irregular papillary structures were seen in dilated gland without stromal invasion, and these cells exhibit marked cellular atypia with mitotic figure (arrow). (c, case 4) (e, case 3) Infiltrative growth of irregular glands (right side) is shown with desmoplastic stroma. Nonatypical small glands (left side) are a part of lobular endocervical glandular hyperplasia. (d, case 4) (f, case 3) Apparent cellular atypia are seen in carcinoma cells with decrease of cytoplasmic mucin.
Histochemistry
The histochemical results are presented in Table 2 . With the alcian blue/periodic acid-Schiff stain, the lobular endocervical glandular hyperplasia components of all cases were revealed as predominantly periodic acid-Schiff-positive neutral mucin, whereas alcian blue positive acid mucin was dominant in the normal endocervical glands (Figure 4a, b). There was less cytoplasmic mucin in adenocarcinoma cells than the lobular endocervical glandular hyperplasia cells, and they had a mixed pattern of alcian blue and periodic acid-Schiff (purple color) (Figure 4c, d).
Histochemistry for alcian blue/periodic acid-Schiff stain. (a) Alcian blue-positive acid mucin is dominant in normal endocervical gland. (b) Periodic acid-Schiff-positive neutral mucin is dominant in glands of lobular endocervical glandular hyperplasia. (c) Purple colored mucin is appeared in adenocarcinoma cells (case 1). (d) Scant alcian blue-positive mucin is found in adenocarcinoma (arrows) (case 4). Benign glands with periodic acid-Schiff positive neutral mucin are a part of lobular endocervical glandular hyperplasia.
Immunohistochemistry
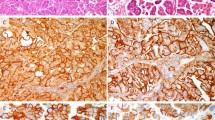
The immunohistochemical results are summarized in Table 3 . Pyloric gland mucin (HIK1083) was diffusely immunopositive in the cytoplasm of lobular endocervical glandular hyperplasia cells (Figure 5a). Focal immunopositivity of HIK1083 was detected in three cases of adenocarcinoma (cases 1–3) (Figure 5b), but was immunonegative in normal endocervical glands. CEA was immunopositive in the cytoplasm of adenocarcinoma cells in three cases (cases 1, 2 and 4) (Figure 5c) and immunonegative in one case (case 3). Nuclear positivities of p53 were found in one case of adenocarcinoma (case 3) (Figure 5d). CEA and p53 were immunonegative in the cells of lobular endocervical glandular hyperplasia and of normal endocervical glands.
Immunohistochemical findings. (a) HIK1083 is diffusely positive in cytoplasm of lobular endocervical glandular hyperplasia cells. (b) Focal immunopositivity of HIK1083 is found in adenocarcinoma cells. (c) CEA is diffusely positive in cytoplasm of adenocarcinoma cells (case 1). (d) Nuclear positivities of p53 are found in adenocarcinoma cells (case 3).
Cytology
In all four cases, cytological examination of cervical smears showed atypical glandular cells with enlarged hyperchromatic nuclei and prominent nucleoli, which were interpreted as adenocarcinoma cells (Figure 6a). In addition, small numbers of glandular cells with golden-yellow mucin were simultaneously detected. These were uniform cells arranged in honeycomb groupings without nuclear stratification (Figure 6c). These benign-looking glandular cells with golden-yellow mucin were suggested to be of lobular endocervical glandular hyperplasia origin. Intranuclear cytoplasmic inclusions also were occasionally found in these glandular cells (Figure 6d). In case 3, we detected adenocarcinoma cells containing scant golden-yellow cytoplasmic mucin (Figure 6b). The cytoplasmic mucin in most of the normal endocervical columnar cells stained orange.
Cytological findings (Papanicolaou stain). (a) Adenocarcinoma cells show disorganized sheets comprised of irregular atypical cells with prominent nucleoli. (b) Scant ‘golden-yellow’ cytoplasmic mucin (arrows) is detected in peripheral of marked crowded sheet of adenocarcinoma cells (case 3). (c) Two color patterns, golden-yellow mucin (left) and orange mucin (right), are found in benign mucinous glandular cells. (d) Intranuclear cytoplasmic inclusions occasionally found in benign-looking glandular cells with golden-yellow mucin (arrows).
Discussion
Lobular endocervical glandular hyperplasia is a pseudoneoplastic lesion of the uterine cervix, however, its progression and pathological significance has not been fully recognized yet. In the current study, we described four cases of endocervical adenocarcinomas coexisting with lobular endocervical glandular hyperplasia.
Histopathologically, lobular endocervical glandular hyperplasia is defined as benign hyperplastic glands arranged in a lobular pattern. They are often confused with minimal deviation adenocarcinoma, so-called adenoma malignum.24, 33 However, minimal deviation adenocarcinoma should be differentiated from lobular endocervical glandular hyperplasia since an extremely poor prognosis has been reported in patients with minimal deviation adenocarcinoma.41, 42, 43 In this rare highly differentiated mucinous adenocarcinoma, occasional glands display moderate nuclear atypia and elicit a desmoplastic stromal reaction, while most of the glands are impossible to distinguish from non-neoplastic endocervical glands including lobular endocervical glandular hyperplasia. According to WHO classification, haphazard arrangements of glands that extend deeply into the cervical stroma and the presence of occasional mitoses are reliable histological criteria for differentiating minimal deviation adenocarcinoma from the various benign endocervical lesions.39 In the current study, all four hyperplastic glandular lesions were composed of benign-looking glands lined by mucin-rich, high columnar cells and arranged in a lobular pattern. There was no desmoplastic stromal reaction, and mitosis was absent in these hyperplastic lesions. These histological findings correspond to the definition of lobular endocervical glandular hyperplasia.44 Nucci et al24 documented the histological differences between lobular endocervical glandular hyperplasia and endocervical tunnel clusters. Our cases were macroscopically multicystic lesions comprised of tall, columnar, mucinous cells. We consider these findings to be different from type A tunnel clusters, which are characterized by noncystic and closely packed lesions composed of tall columnar epithelium, and from type B tunnel clusters, which are characterized by lobular arrangements with cystic glands lined by cuboidal and flattened epithelial cells with inspisated mucinous secretions.20, 21, 22
In our study, we also found four mucinous endocervical adenocarcinomas coexisting with lobular endocervical glandular hyperplasia: three were classified as invasive adenocarcinomas and one was adenocarcinoma in situ. Immunopositivities of CEA or p53 further supported the diagnosis of adenocarcinoma, while these same markers were immunonegative in the lobular endocervical glandular hyperplasia tissue. In general, the majority of conventional adenocarcinoma in situ and invasive adenocarcinomas involve the transformation zone of the cervix.45, 46, 47 However, all four adenocarcinomas in our study were detected more proximally along the cervix and did not involve the transformation zone. These findings may indicate that adenocarcinomas in our four cases are unusual in their location. To the best of our knowledge, the current study is the first report on endocervical adenocarcinoma associated with lobular endocervical glandular hyperplasia.
Histological assessment revealed that glandular cells of lobular endocervical glandular hyperplasia are rich in mucin, and golden-yellow cytoplasmic mucins, an unusual color in normal endocervical glands, were detected in Pap smears. In addition, an increase of watery vaginal discharge was a presenting complaint of two patients. Consequently, we looked at the mucin characteristics of endocervical lesions and then performed immunohistochemistry for pyloric gland mucin (HIK1083) and staining of alcian blue/periodic acid-Schiff. Diffuse immunopositivities of HIK1083 and predominantly periodic acid-Schiff-positive neutral mucin expression were recognized in the glands of the lobular endocervical glandular hyperplasia, corresponding with the observations of previous investigators.29, 33, 34
HIK1083-positive pyloric gland mucin was focally evident in three of four adenocarcinomas. This common expression of pyloric gland mucin in both lobular endocervical glandular hyperplasia and mucinous endocervical adenocarcinomas suggests the possibility that there is a histogenetical relationship between these two lesions. However, this needs to be studied further because Zhao et al48 reported HIK1083 positivity in a variety of cervical adenocarcinomas, and no one has yet determined the frequency of HIK1083 positivity in the wide variety of other benign glandular lesions.
In the uterine cervix, pyloric gland mucin phenotype has been reported in endocervical gastric metaplasia, lobular endocervical glandular hyperplasia and minimal deviation adenocarcinoma.27, 29, 30, 32, 33, 34, 36 Because of the identical mucin expression in these lesions, several investigators have suggested that metaplastic or hyperplastic glandular lesions with pyloric gland mucin could be a precursor of minimal deviation adenocarcinoma.32, 34, 37 Pyloric gland metaplasia also has been described in several other organs such as the pancreas, the gallbladder and the small and large intestine.49, 50, 51, 52 Pathogenesis of this metaplastic change in most organs is still under discussion; however, its relationship to carcinogenesis in the pancreas has received particular attention. In the pancreas, pyloric gland metaplasia is a common histological change of the pancreatic duct, and Kodama and Mori53 have suggested that this metaplastic lesion might transform into atypical proliferations and then into adenocarcinomas. Recent WHO classification proposed that the term ‘pancreatic intraepithelial neoplasia’ (PanIN) should be used for several metaplastic or hyperplastic lesions, including pyloric gland metaplasia of the pancreatic duct, as a putative precursor lesion of invasive ductal adenocarcinoma.54, 55 With this information, and from our results, it is reasonable to speculate that lobular endocervical glandular hyperplasia with pyloric gland metaplasia is not only a precursor of minimal deviation adenocarcinoma but also a possible precursor of conventional mucinous adenocarcinoma.
Cytologically, golden-yellow cytoplasmic mucin was found in benign-looking endocervical glandular cells. This is congruent with the finding of lobular endocervical glandular hyperplasia reported by Hata et al.56 This distinctive color of cytoplasmic mucin may reflect the pyloric phenotype of glandular cells, and taking into account the fact that endocervical adenocarcinomas were coexisting with lobular endocervical glandular hyperplasia in the current study, we recommend careful cytological assessment when this golden-yellow mucin is detected in Pap smears, even if cellular atypia is not apparent in the sample. However, it will be difficult to differentiate lobular endocervical glandular hyperplasia from minimal deviation adenocarcinoma solely based on mucin colors since golden-yellow cytoplasmic mucin also has been suggested as representing the cytological features of minimal deviation adenocarcinoma.57
We found intranuclear cytoplasmic inclusions in the lobular endocervical glandular hyperplasia cells in all four cases, but did not detect them in cancer cells. Since we have found no reports of these inclusions in other glandular lesions of the uterine cervix, this nuclear finding might be one of the cytological characteristics of lobular endocervical glandular hyperplasia. Intranuclear cytoplasmic inclusions have been described in certain benign and malignant neoplasms such as melanocytic nevi, malignant melanomas and pulmonary adenocarcinomas, and the diagnostic value of this type of inclusion are especially important in thyroid papillary carcinomas.58, 59, 60 However, pathological and diagnostic implications of this finding in lobular endocervical glandular hyperplasia are obscure.
Infection by the human papilloma virus (HPV) is the major etiological factor for both cervical squamous cell carcinoma and cervical adenocarcinoma,61, 62, 63 although the pathological association of HPV and lobular endocervical glandular hyperplasia have not been well studied. At least, our PCR analysis did not detect HPV in lobular endocervical glandular hyperplasia cells (in press). Further investigation of HPV infection and genetic abnormalities is needed to clarify the etiologies of endocervical adenocarcinoma associated with lobular endocervical glandular hyperplasia.
In our clinical data, three patients in early stages (UICC stage 0 and Ia1) showed no metastasis and no local recurrence during our limited follow-up period, while one patient (case 4, stage IIIb) died due to lung metastasis 13 months after surgery. In general, the prognosis of microinvasive adenocarcinoma (stage Ia) and adenocarcinoma in situ (stage 0) is excellent in conventional endocervical adenocarcinoma. The clinical course of our cases was similar to the course of conventional endocervical adenocarcinoma without lobular endocervical glandular hyperplasia. At this time, the pathological effect that the lobular endocervical glandular hyperplasia has on the prognosis of endocervical adenocarcinoma is indistinct.
In our study, we reviewed 24 cases of lobular endocervical glandular hyperplasia in the files of affiliated hospitals from 1996 to 2003 and retrieved four cases, which we presented here. However, the prevalence of lobular endocervical glandular hyperplasia in our study may be high compared with the general prevalence, since lobular endocervical glandular hyperplasia is considered to be a rare lesion.37 There may be several reasons to explain the high prevalence of lobular endocervical glandular hyperplasia in our facilities. First, more pathologists, gynecologists and radiologists are beginning to recognize this rare lesion more often. Second, confusion on the diagnosis of benign hyperplastic lesions and minimal deviation adenocarcinoma might elicit an increased number of lobular endocervical glandular hyperplasia diagnoses. Since Ishii and co-workers30, 32, 57 described the cytological and histological features of minimal deviation adenocarcinoma, we have actively interpreted golden-yellow mucin in Pap smears and gastric mucin in histological specimens (findings are now thought to be nonspecific for minimal deviation adenocarcinoma) as indicators of minimal deviation adenocarcinoma. Third, for the purpose of finding the early stage of minimal deviation adenocarcinoma, we tested a vaginal discharge screening test using enzyme-linked immunosorbent assay for HIK1083 in gynecology outpatients. Under these conditions, surgical specimens of endocervical glandular lesions with gastric phenotype, including lobular endocervical glandular hyperplasia, increased during the period of our study.
The exact prevalence of adenocarcinomas coexisting with lobular endocervical glandular hyperplasia among cervical adenocarcinomas is unclear. From a practical standpoint, to recognize a residual component of lobular endocervical glandular hyperplasia will be difficult when invasive adenocarcinoma takes over large proportions of the lesion. During routine diagnostic work, if we found benign-looking minute glandular lesions with gastric phenotype intermingled with an invasive adenocarcinoma, we could not differentiate these minute lesions as being a residual part of lobular endocervical glandular hyperplasia or small foci of a very well-differentiated adenocarcinoma. Molecular analysis may be helpful in differentiating these lesions, but this distinction will be less meaningful for the patient's management since the poorly differentiated malignant component is generally what affects the clinical course.
In conclusion, this report provides new information on endocervical adenocarcinoma associated with lobular endocervical glandular hyperplasia. We emphasize that careful examination is needed in the histological diagnosis of lobular endocervical glandular hyperplasia to exclude the coexistence of adenocarcinomas, and a focal adenocarcinoma coexisting with lobular endocervical glandular hyperplasia should be differentiated from minimal deviation adenocarcinoma. In addition, it is reasonable to suppose that lobular endocervical glandular hyperplasia could be a precursor of conventional mucinous endocervical adenocarcinomas based on histopathological features of the present cases.
References
Hopkins MP, Morley GW . A comparison of adenocarcinoma and squamous cell carcinoma of the cervix. Obstet Gynecol 1991;77:912–917.
Kjaer SK, Brinton LA . Adenocarcinomas of the uterine cervix: the epidemiology of an increasing problem. Epidemiol Rev 1993;15:486–498.
Shingleton HM, Gore H, Bradley DH, et al. Adenocarcinoma of the cervix. I. Clinical evaluation and pathologic features. Am J Obstet Gynecol 1981;139:799–814.
Vizcaino AP, Moreno V, Bosch FX, et al. International trends in the incidence of cervical cancer: I. Adenocarcinoma and adenosquamous cell carcinomas. Int J Cancer 1998;75:536–545.
Brown LJ, Wells M . Cervical glandular atypia associated with squamous intraepithelial neoplasia: a premalignant lesion? J Clin Pathol 1986;39:22–28.
Casper GR, Ostor AG, Quinn MA . A clinicopathologic study of glandular dysplasia of the cervix. Gynecol Oncol 1997;64:166–170.
Farnsworth A, Laverty C, Stoler MH . Human papillomavirus messenger RNA expression in adenocarcinoma in situ of the uterine cervix. Int J Gynecol Pathol 1989;8:321–330.
Gloor E, Hurlimann J . Cervical intraepithelial glandular neoplasia (adenocarcinoma in situ and glandular dysplasia). A correlative study of 23 cases with histologic grading, histochemical analysis of mucins, and immunohistochemical determination of the affinity for four lectins. Cancer 1986;58:1272–1280.
Goldstein NS, Ahmad E, Hussain M, et al. Endocervical glandular atypia: does a preneoplastic lesion of adenocarcinoma in situ exist? Am J Clin Pathol 1998;110:200–209.
Jaworski R . Endocervical glandular dysplasia, adenocarcinoma in situ and nearly invasive (microinvasive) adenocarcinoma of the uterine cervix. Semin Diagn Pathol 1990;7:190–204.
Kurian K, al-Nafussi A . Relation of cervical glandular intraepithelial neoplasia to microinvasive and invasive adenocarcinoma of the uterine cervix: a study of 121 cases. J Clin Pathol 1999;52:112–117.
Moritani S, Ioffe OB, Sagae S, et al. Mitotic activity and apoptosis in endocervical glandular lesions. Int J Gynecol Pathol 2002;21:125–133.
Schlesinger C, Silverberg SG . Endocervical adenocarcinoma in situ of tubal type and its relation to atypical tubal metaplasia. Int J Gynecol Pathol 1999;18:1–4.
Chumas JC, Nelson B, Mann WJ, et al. Microglandular hyperplasia of the uterine cervix. Obstet Gynecol 1985;66:406–409.
Leslie KO, Silverberg SG . Microglandular hyperplasia of the cervix: unusual clinical and pathological presentations and their differential diagnosis. Prog Surg Pathol 1984;5:95–114.
Wilkinson E, Dufour DR . Pathogenesis of microglandular hyperplasia of the cervix uteri. Obstet Gynecol 1976;47:189–195.
Young RH, Scully RE . Atypical forms of microglandular hyperplasia of the cervix simulating carcinoma. A report of five cases and review of the literature. Am J Surg Pathol 1989;13:50–56.
Ferry JA, Scully RE . Mesonephric remnants, hyperplasia and neoplasia in the uterine cervix. A study of 49 cases. Am J Surg Pathol 1990;14:1100–1111.
Seidman JD, Tavassoli FA . Mesonephric hyperplasia of the cervix: a clinicopathological study of 51 cases. Int J Gynecol Pathol 1995;14:293–299.
Flumann CF . Focal hyperplasia (tunnel cluster) of the cervix uteri. Obstet Gynecol 1961;17:206–214.
Jones MA, Young RH . Endocervical type A (noncystic) tunnel clusters with cytologic atypia. A report of 14 cases. Am J Surg Pathol 1996;20:1312–1318.
Segal GH, Hart WR . Cystic endocervical tunnel clusters. A clinicopathologic study of 29 cases of so-called adenomatous hyperplasia. Am J Surg Pathol 1990;14:895–903.
Jones MA, Young RH, Scully RE . Diffuse laminar endocervical glandular hyperplasia. A benign lesion often confused with adenoma malignum (minimal deviation adenocarcinoma). Am J Surg Pathol 1991;15:1123–1129.
Nucci MR, Clement PB, Young RH . Lobular endocervical glandular hyperplasia, not otherwise specified: a clinicopathologic analysis of thirteen cases of distinctive pseudoneoplastic and comparison with fourteen cases of adenoma malignum. Am J Surg Pathol 1999;23:886–891.
Jones MW, Silverberg SG . Cervical adenocarcinoma in young women: possible relationship to microglandular hyperplasia and use of oral contraceptives. Obstet Gynecol 1989;73:984–989.
Tase T, Okagaki T, Clark BA, et al. Human papillomavirus DNA in glandular dysplasia and microglandular hyperplasia: presumed precursors of adenocarcinoma of the uterine cervix. Obstet Gynecol 1989;73:1005–1008.
Bulmer JN, Griffin NR, Bates C, et al. Minimal deviation adenocarcinoma (adenoma malignum) of the endocervix: a histochemical and immunohistochemical study of two cases. Gynecol Oncol 1990;36:139–146.
Fand SB . The histochemistry of human cervical endometrium. In: Blandau RJ, Moghissi K (eds). The Biology of the Cervix. University of Chicago Press: Chicago, 1973, pp 103–124.
Hayashi I, Tsuda H, Shimoda T, et al. Difference in cytoplasmic localization pattern of neutral mucin among lobular endocervical glandular hyperplasia, adenoma malignum, and common adenocarcinoma of the uterine cervix. Virchows Arch 2003;443:752–760.
Hayashi I, Tsuda H, Shimoda T . Reappraisal of orthodox histochemistry for the diagnosis of minimal deviation adenocarcinoma of the cervix. Am J Surg Pathol 2000;24:559–562.
Ishihara K, Kurihara M, Goso, Y, et al. Peripheral alpha-linked N-acetylglucosamine on the carbohydrate moiety of mucin derived from mammalian gastric gland mucous cells: epitope recognized by a newly characterized monoclonal antibody. Biochem J 1996;318:409–416.
Ishii K, Hosaka N, Toki T, et al. A new view of the so-called adenoma malignum of the uterine cervix. Virchows Arch 1998;432:315–322.
Mikami Y, Hata S, Fujiwara K, et al. Florid endocervical glandular hyperplasia with intestinal and pyloric gland metaplasia: worrisome benign mimic of ‘adenoma malignum’. Gynecol Oncol 1999;74:504–511.
Mikami Y, Hata S, Melamed J, et al. Lobular endocervical glandular hyperplasia is a metaplastic process with a pyloric gland phenotype. Histopathology 2001;39:364–372.
Ota H, Hayama M, Nakayama J, et al. Cell lineage specificity of newly raised monoclonal antibodies against gastric mucin in normal, metaplastic, and neoplastic human tissues and their application to pathology diagnosis. Am J Clin Pathol 2001;115:69–79.
Toki T, Shiozawa T, Hosaka N, et al. Minimal deviation adenocarcinoma of the uterine cervix has abnormal expression of sex steroid receptors, CA125, and gastric mucin. Int J Gynecol Pathol 1997;16:111–116.
Tsuda H, Okada S, Kasamatsu T . Is florid pyloric metaplasia different from adenoma malignum? Gynecol Oncol 2000;77:341–343.
Sobin LH, Wittekind CH, (eds). TNM Classification of Malignant Tumors, 6th edn. Wiley-Liss: New York, 2002.
Tavassoli FA, Devilee P, (eds). World Health Organization Classification of Tumors, Tumor of the Breast and Female Genital Organs. IARC Press: Lyon, 2003.
Spicer SS, Horn RG, Leppi TJ . Histochemistry of connective tissue mucopolysaccharides In: Wagner BM, Smith DE (eds). The Connective Tissue. Williams & Wilkins: Baltimore, 1976, pp 251–303.
Silverberg SG, Hurt WG . Minimal deviation adenocarcinoma (‘adenoma malignum’) of the cervix: a reappraisal. Am J Obstet Gynecol 1975;121:971–975.
McKelvey JL, Goodlin RR . Adenoma malignum of the uterine cervix. A cancer of deceptively innocent histological pattern. Cancer 1963;16:549–557.
Kaku T, Enjoji M . Extremely well-differentiated adenocarcinoma (‘adenoma malignum’) of the cervix. Int J Gynecol Pathol 1983;2:28–41.
Nucci MR . Symposium Part III: tumor-like glandular lesions of the uterine cervix. Int J Gynecol Pathol 2002;21:347–359.
Andersen ES, Arffmann E . Adenocarcinoma in situ of the uterine cervix: a clinico-pathologic study of 36 cases. Gynecol Oncol 1989;35:1–7.
Bertrand M, Lickrish GM, Colgan TJ . The anatomic distribution cervical adenocarcinoma in situ: implications for treatment. Am J Obstet Gynecol 1987;157:21–25.
Teshima S, Shimosato Y, Kishi K, et al. Early stage adenocarcinoma of the cervix. Histopathologic analysis with consideration of histogenesis. Cancer 1985;56:167–172.
Zhao S, Hayasaka T, Osakabe M, et al. T Mucin expression in nonneoplastic and neoplastic glandular epithelia of the uterine cervix. Int J Gynecol Pathol 2003;22:393–397.
Albores-Saavedra J, Henson DE . Pyloric gland metaplasia with perineural invasion of the gallbladder: A lesion that can be confused with adenocarcinoma. Cancer 1999;86:2625–2631.
Lee FD . Pyloric metaplasia in the small intestine. J Pathol Bacteriol 1964;87:267–277.
Tatematsu M, Furihata C, Miki K, et al. Complete and incomplete pyloric gland metaplasia of human gallbladder. Acta Pathologica Japonica 1987;37:39–46.
Yokoyama I, Kozuka S, Ito K, et al. Gastric gland metaplasia in the small and large intestine. Gut 1977;18:214–219.
Kodama T, Mori W . Morphological lesions of the pancreatic ducts. Significances of pyloric gland metaplasia in carcinogenesis of exocrine and endocrine pancreas. Acta Pathologica Japonica 1983;33:645–660.
Hamilton SR, Aaltonen LA, (eds). World Health Organization Classification of Tumors, Tumor of the Digestive System. IARC Press: Lyon, 2000.
Hruban RH, Adsay NV, Albores-Saavedra J, et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol 2001;25:579–586.
Hata S, Mikami Y, Manabe T . Diagnostic significance of endocervical glandular cells with ‘golden-yellow’ mucin on Pap smear. Diagn Cytopathol 2002;27:80–84.
Ishii K, Katsuyama T, Watanabe T, et al. Cytological and cytochemical features of adenoma malignum of the uterine cervix. Cancer 1999;87:245–253.
Hedinger C, Williams ED, Sobin LH, (eds). Histological Typing of Thyroid Tumors. International Histological Classification of Tumours, World Health Organization, 2nd edn. Springer-Verlag: Berlin, 1988.
Hiroshima K, Toyozaki T, Iyoda A, et al. Ultrastructural study of intranuclear inclusion bodies of pulmonary adenocarcinoma. Ultrastruct Pathol 1999;23:383–389.
Tsumuraya M, Kodama T, Kameya T, et al. Light and electron microscopic analysis of intranuclear inclusions in papillary adenocarcinoma of the lung. Acta Cytol 1981;25:523–532.
Ferguson AW, Svoboda-Newman SM, et al. Analysis of human papillomavirus infection and molecular alterations in adenocarcinoma of the cervix. Mod Pathol 1998;1:11–18.
Pirog EC, Kleter B, Olgac S, et al. Prevalence of human papillomavirus DNA in different histological subtypes of cervical adenocarcinoma. Am J Pathol 2000;157:1055–1062.
Wilczynski SP, Walker J, Liao SY, et al. Adenocarcinoma of the cervix associated with human papillomavirus. Cancer 1988;62:1331–1336.
Acknowledgements
We thank Dr T Oyama and Dr K Teramoto, Yamanashi Central Hospital, Japan, and Dr K Miyata and Dr K Takaishi, Kofu Municipal Hospital, Japan, for providing the case materials and giving clinical information, and also Dr T Moriya, Tohoku University, Japan, for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kondo, T., Hashi, A., Murata, Si. et al. Endocervical adenocarcinomas associated with lobular endocervical glandular hyperplasia: a report of four cases with histochemical and immunohistochemical analyses. Mod Pathol 18, 1199–1210 (2005). https://doi.org/10.1038/modpathol.3800403
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800403
Keywords
This article is cited by
-
Trefoil factor family 2 protein: a potential immunohistochemical marker for aiding diagnosis of lobular endocervical glandular hyperplasia and gastric-type adenocarcinoma of the uterine cervix
Virchows Archiv (2019)
-
Clonality analysis suggests that STK11 gene mutations are involved in progression of lobular endocervical glandular hyperplasia (LEGH) to minimal deviation adenocarcinoma (MDA)
Virchows Archiv (2013)