Abstract
Recent changes in the classification of cervical adenocarcinomas have re-categorized serous carcinoma as potentially nonexistent. However, clinical and pathological profiles of cervical adenocarcinomas with serous-like morphological features have not been systematically evaluated using the latest taxonomy and biomarkers. We studied 14 cases of primary cervical carcinomas with serous-like morphologies (papillary and micropapillary patterns). None of these cases exhibited evidence of serous carcinoma involving the upper tracts. Patient ages ranged between 34 and 86 years, most presented with abnormal uterine bleeding. Histologically, ten cases were classified as human papillomavirus (HPV)-associated carcinomas (eight usual-type endocervical adenocarcinomas and two adenosquamous carcinomas), of which six exhibited a papillary pattern and four had a micropapillary pattern. The four remaining cases were HPV-independent gastric-type adenocarcinomas, which displayed a papillary pattern in one case and a micropapillary pattern in three others. All ten HPV-associated carcinomas displayed block positive p16 and wild-type p53 by immunohistochemistry, with nine of them confirmed by HPV testing. Two of the four gastric-type adenocarcinomas had mutation-type p53, one of which also being p16 block positive. HER2 overexpression was demonstrated in 3/14 (21.4%) cases (2 HPV-associated and 1 HPV-independent). PD-L1 expression was identified in 4/10 (40%) cases, all HPV-associated. Targeted next-generation sequencing was performed in two cases with a micropapillary pattern, revealing a missense variant in ATM in an HPV-associated tumor and missense variants in TP53 and SMARCB1 in an HPV-independent tumor. The results demonstrated that primary endocervical adenocarcinomas can mimic the appearance of serous carcinoma, while not representing serous carcinoma. Serous-like papillary and micropapillary patterns may be present in both HPV-associated and HPV-independent cervical carcinomas, but none of the cases studied were unequivocally serous upon detailed analysis. Our findings support the exclusion of “cervical serous carcinoma” from existing classifications of cervical adenocarcinoma.
Similar content being viewed by others
Introduction
Serous carcinoma of the uterine cervix was included as a histologic subtype of cervical adenocarcinoma in the 2014 World Health Organization (WHO) classification [1] and the International Endocervical Adenocarcinoma Criteria and Classification (IECC) [2, 3]. It is characterized by the morphological features of complex papillary and/or micropapillary structures with high-grade nuclear atypia. With the current paradigm shift toward an etiology-based classification of cervical adenocarcinomas [2, 4], it has been suggested that primary cervical serous carcinomas probably do not occur [3,4,5] and thus will be excluded from the upcoming 2020 WHO classification. Most reported cases are believed to represent either human papillomavirus (HPV)-associated adenocarcinomas with serous-like patterns, endometrial extensions, or tubo-ovarian serous carcinomas [2,3,4,5,6]. On the other hand, several recent studies have reported the pathological profiles and clinical significance of cervical carcinomas with micropapillary patterns [7,8,9,10], which are often associated with HPV-associated adenocarcinomas, and carry adverse prognostic implications. It is currently unknown if cervical carcinomas with micropapillary patterns represent a biologically distinct entity, and their molecular pathogenesis has not been elucidated. With the increasing recognition of the morphological spectrum of cervical gastric-type adenocarcinomas, some cases have documented the presence of serous-like papillary areas [11]. To our knowledge, very few cases of HPV-independent cervical adenocarcinomas displaying serous-like features have been reported [10] and systematic studies have not been undertaken. There is a need for updated evidence regarding the clinical and pathological implications of serous-like morphological features in cervical adenocarcinomas with regard to the current concepts and diagnostic/predictive markers.
In view of the nosologic conundrum concerning these tumors, we studied a series of cervical carcinomas with a serous-like papillary and/or micropapillary pattern. With the aid of immunohistochemistry, HPV testing, and molecular analysis, we attempted to evaluate the relevance of these serous-like morphological features within the current classification schema.
Materials and methods
After ethics approvals were obtained (IRB numbers: HKECREC-2018-023 and UW 18–293), cervical carcinomas diagnosed as “adenocarcinoma,” “adenosquamous carcinoma,” and “serous carcinoma” were identified by searching the pathology databases between 1994 and 2019 from one institution, and between 2000 and 2019 from another.
Clinical history and follow-up data were obtained from the clinical records. All were histologically reviewed by gynecological pathologists. After the exclusion of secondary involvement by serous carcinoma from the corpus and upper tracts, they were classified into HPV-associated or HPV-independent carcinomas based on IECC criteria. HPV-associated carcinoma was defined by the presence of prominent apical mitoses and apoptotic bodies at scanning magnification whereas cases lacking these features were classified as HPV-independent carcinomas [2, 3]. These cervical carcinomas were then considered to have “serous-like features” if they contain ≥ 5% of serous-like papillary pattern or a micropapillary pattern, as defined by Stewart et al. for micropapillary components in cervical carcinomas [9]. The papillary pattern was characterized by a complex papillary architecture with cellular budding and high-grade nuclear atypia (moderate to marked nuclear pleomorphism, increased nuclear-cytoplasmic ratio and prominent nucleoli), based on the 2014 WHO classification [1]. Papillae of the villoglandular type were distinguished from serous-like papillae by their exophytic long slender architecture and mild to moderate cytological atypia [1, 2]; cases with pure villoglandular features were not included. The micropapillary pattern was defined as groups of tumor cells without fibrovascular cores within cleft-like or lacunar spaces and exhibiting high-grade nuclear atypia, and have classical immunohistochemical staining pattern with EMA, MUC1, E-cadherin, and p120-catenin [9, 10]. A micropapillary pattern must show membrane staining on the peripheral borders of tumor clusters with EMA and/or MUC1, together with absence of peripheral border staining and preserved lateral membrane staining with E-cadherin and/or p120-catenin [9] (Fig. 1). All four markers were performed on cases with morphological suspicion of a micropapillary pattern, while those lacking immunohistochemical confirmation were excluded.
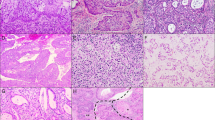
Micropapillary component is morphologically defined as groups of tumor cells without fibrovascular cores present within empty cleft-like spaces (a, b). The key feature of reversed epithelial polarity is immunohistochemically visualized as membrane staining for EMA (c) and/or MUC1 (d) on the peripheral cell borders, together with absent peripheral border staining and intact lateral membrane staining with E-cadherin (e) and/or p120-catenin (f).
Immunohistochemical analysis was performed for all cases except for PD-L1, which was tested in ten cases (see Supplementary Table 1). Markers included p16, p53, MUC6, CEA, PAX8, estrogen receptor (ER), CK7, CK20, WT1, CDX2, GATA3, p40, HER2, and PD-L1 (22C3). With the exception of p16, p53, HER2, and PD-L1, staining for all other markers was recorded as diffusely positive (≥50% cells staining), focally positive (<50% cells staining), or negative. p16 staining was recorded as block-type positive (positive nuclear and cytoplasmic staining of all tumor cells) or negative (negative or “non-block-type” staining). p53 staining was recorded as “mutation-type” (diffuse strong positive staining in >80% of cells, completely negative, or cytoplasmic staining) or “wild-type” (heterogeneous staining of variable intensity in <80% of cells) [12]. HER2 immunohistochemistry was classified as positive (3+), equivocal (2+), or negative (1+ or 0), based on the 2018 American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update for HER2 testing in breast cancer [13]. PD-L1 immunohistochemistry was assessed using the combined positive score (CPS; defined as total number of positive tumor cells and immune cells/total number of viable tumor cells × 100) and classified into negative (CPS < 1) or positive expression (CPS ≥ 1) [14].
HPV testing was performed on DNA samples extracted from paraffin blocks in 12 cases with adequate material by polymerase chain reaction using the consensus primers GP5+/6+, followed by typing by Sanger sequencing for positive cases. Targeted next-generation sequencing (NGS) was successfully performed in two cases with a micropapillary pattern (cases 6 and 14) using DNA extracted from formalin-fixed and paraffin-embedded tissue with microdissection of the area with the micropapillary features. Paired normal samples were not examined. Library preparation and targeted NGS for 207 amplicons covering mutational hotspot regions in 50 cancer-related genes (listed in Supplementary Table 2) was performed using the Ion Ampliseq Cancer Hotspot Panel v2 on the Ion S5 sequencer following the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA) and as previously described [15]. Sequencing data were processed using the Torrent Suite software v5.6.0 and analyzed using the Ion Reporter software v5.10.1.0 (Thermo Fisher Scientific) with mapping to the reference GRCh37 (hg19). The minimum accepted vertical coverage was 1000× for detection of ≥5% variant allele frequency. Copy number variation was not assessed. The variants identified were individually evaluated to exclude known polymorphisms and assess the significance by reviewing published literature, and the ClinVar and Catalogue of Somatic Mutations in Cancer (COSMIC) databases.
Results
Among the 136 cases of primary cervical carcinomas, 14 (10.3%) that met all the criteria were included in the study. The original diagnosis was “adenocarcinoma” in seven cases, “serous adenocarcinoma” in two cases, while the remaining five were diagnosed as “papillomatous adenocarcinoma,” “micropapillary carcinoma,” “usual endocervical adenocarcinoma,” “mucinous adenocarcinoma,” and “adenosquamous carcinoma,” respectively. All the slides containing tumor were re-reviewed to evaluate the histologic types and other pathological features. For all included cases, there was no evidence of serous carcinoma in the upper genital tract based on hysterectomy (nine cases) and salpingo-oophorectomy (eight cases) specimens if available, as well as review of concurrent endometrial biopsies, immunohistochemical profiles and radiological investigations.
The number of slides available for review ranged from 1 to 12 per case (7 on average). The types of specimens included hysterectomy (nine cases), loop electrosurgical excision procedure (one case) and biopsies (four cases).
Cervical carcinomas with serous-like morphologies included in the study comprised of ten HPV-associated carcinomas (eight usual-type adenocarcinomas and two adenosquamous carcinomas) and four HPV-independent (gastric type) carcinomas. Six other cases with suspicion of a micropapillary pattern (presence of tumor cell clusters without fibrovascular cores surrounded by empty spaces), but without typical immunohistochemical confirmation (none of them had any of the typical expression patterns for EMA, MUC1, E-cadherin, and p120-catenin) were excluded.
Clinical features
Details of the clinical features are listed in Table 1.
The age of ten patients with HPV-associated carcinomas ranged from 34 to 62 years (mean, 48.3 years). Eight (80%) presented with abnormal uterine bleeding. The tumor size ranged from 2 to 7 cm (mean, 4.5 cm). In eight cases, the tumor was grossly visible and their locations were documented. Four of these were localized to either the anterior or posterior lip and histologically, they all had a papillary pattern. With the remainder four that had circumferential cervical involvement, three contained a micropapillary pattern and one had a papillary pattern. The tumors were FIGO (2018) stage I in five cases, stage II in one case, stage III in three cases, and stage IV in one case. The six stages I/II cases all had a hysterectomy and, with the exception of case 5 (stage IA), a pelvic lymph node dissection was also performed. After a mean follow-up of 5.75 years, four were alive with no evidence of disease. Two patients had vaginal vault recurrence respectively at 1 and 3 years after the initial operation, and both were successfully treated with chemotherapy and radiotherapy. In the four remaining patients, two patients died of disease at 4 months and 2 years, respectively, one patient was alive with progressive disease at 7 years, and one had no follow-up available. The 5-year overall survival (excluding cases 9 and 10) was 75% (6/8).
The age of the four patients with HPV-independent carcinomas ranged from 45 to 86 years (mean, 66.3 years). Three of the four presented with postmenopausal bleeding. The tumor size ranged from 1.6 to 5.7 cm (mean, 3.5 cm). For the three cases with their locations documented, one was localized to the anterior lip and was found to have a papillary pattern, while two others that involved the entire cervix both had a micropapillary pattern. Two cases were FIGO stage I while the other two were stage III at presentation. Two of the four had a hysterectomy, one of which also had pelvic lymph node dissection. Upon follow-up, three patients died of disease after 12 months, 20 months, and 4 years, respectively, with invasion of adjacent organs or distant metastasis, while the remaining patient showed no evidence of disease at 2 years. The 5-year overall survival (excluding case 14) was 0% (0/3).
Pathological features
Details of the pathological features are listed in Table 2. Among all the tumors, these serous-like papillary or micropapillary patterns accounted for 10–100% of the tumor areas, with a mean of 62.1%, which was well over the definitional threshold of 5%. None of the cases demonstrated both papillary and micropapillary patterns simultaneously.
Of the ten HPV-associated carcinomas, eight were usual-type endocervical adenocarcinomas; five of these had a papillary pattern and three had a micropapillary pattern. One of the two adenosquamous carcinomas had a papillary pattern and the other had a micropapillary pattern.
In cases in which the infiltrative pattern was assessable, two of those with papillary pattern were Silva pattern B, and three were pattern C. All four tumors with a micropapillary pattern were Silva pattern C. Lymphovascular invasion was present in 3 of 8 HPV-associated carcinomas and both of 2 gastric-type adenocarcinomas with resection specimens available. The histological appearance and immunohistochemical findings are illustrated in Figs. 2 and 3.
A case of HPV-associated adenocarcinoma features complex branching papillary structures with tufted luminal borders (a) as well as high-grade nuclear atypia and frequent mitoses (b), reminiscent of serous carcinoma. Some HPV-associated cases may be predominantly exophytic and associated with adenocarcinoma in situ (c), with slit-like lumina among tumor cells over papillary structures (d). HPV-associated adenocarcinomas with micropapillary component may be accompanied by HSIL (e) and some cases may harbor tumor cells with squamoid appearance as illustrated here (f).
Complex papillary structures composed of neoplastic squamous and glandular cells are present in a tumor (case 9) (a, b). In another tumor (case 10), areas of typical micropapillary features (left) coexist with larger groups of carcinoma cells without fibrovascular cores (right) (c). At high power, these solid structures comprise a peripheral rim of glandular tumor cells surrounding non-keratinizing squamous tumor cells in the center (d), demonstrated by p40 immunohistochemistry as diffuse staining of the central part and negative at the periphery (e). HER2 overexpression is observed in the micropapillary component (right) contrasting with negative membrane staining of the HSIL element (lower left) (f).
The four HPV-independent carcinomas were all gastric-type adenocarcinomas. One of these had a papillary pattern and three had a micropapillary pattern. The histological features of the HPV-independent (gastric type) adenocarcinomas are illustrated in Fig. 4.
Gastric-type adenocarcinoma may exhibit papillary structures as shown here (a), in which the morphological features of gastric-type differentiation such as abundant foamy cytoplasm and distinct cell borders may be encountered (b). Some cases of gastric-type adenocarcinoma may show typical glandular structures coexisting with micropapillary foci (arrows) (c), which exhibit tumor cell clusters within empty spaces (d).
Immunohistochemistry and HPV testing
The immunohistochemical findings are listed in Table 3.
All ten HPV-associated carcinomas were diffusely block positive for p16 and had a wild-type pattern for p53. These tumors were also positive for CK7 (10/10), CEA (10/10), and PAX8 (9/10). Focal expression of GATA3 and p40 was noted in two and four cases, respectively (concurrently in two cases). CDX2 and WT1 were negative in all ten cases. HER2 was positive (3+) in two cases, equivocal (2+) in one case (for which dual-probe in situ hybridization was negative for HER2 amplification), and negative (0 or 1+) in seven cases. PD-L1 showed positive expression in 4 of 8 cases tested. The HPV test was positive in all of the nine cases with available material for testing (type 16 in 4 and type 18 in 5).
In the HPV-independent gastric-type adenocarcinomas, the only case with papillary pattern showed diffuse block staining for p16 but also demonstrated mutation-type p53 (overexpression). The remaining three gastric-type adenocarcinomas with micropapillary components were negative for p16; one of them had mutation-type p53. All four cases were positive for CK7, CEA, CK20, and MUC6, while negative for ER, GATA3, p40, and WT1. HER2 was positive (3+) in one case and negative (0 or 1+) in the other three. PD-L1 showed negative expression in both of the two cases tested. HPV was confirmed to be negative in three cases with adequate material for testing.
The immunohistochemical features of the HPV-associated tumors and those of the HPV-independent tumors are illustrated in Figs. 5 and 6, respectively.
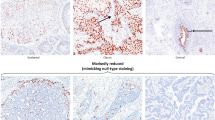
HPV-associated adenocarcinomas typically show diffuse positivity for p16 (a) and wild-type pattern for p53 (b). GATA3 expression is present in both the micropapillary component and accompanying HSIL (left) in case 6 (c), which also exhibits focal p40 expression in the micropapillary areas (d). Overexpression of HER2 is observed in a case of HPV-associated adenocarcinoma with papillary pattern (e). PD-L1 expression in the micropapillary component is visualized as patchy weak to moderate membrane staining in some cases (f).
Gastric-type adenocarcinoma with papillary pattern may exhibit mutation-type overexpression of p53 (a) and focal MUC6 expression (b). Gastric-type adenocarcinomas with micropapillary pattern sometimes display expression of gastrointestinal markers, such as MUC6 (c) and CK20 (d). Mutation-type p53 expression is identified in the micropapillary component of a gastric-type adenocarcinoma (e). HER2 overexpression may manifest as intense complete basolateral membrane staining of tumor cells in gastric-type adenocarcinoma with papillary pattern (f).
Targeted NGS
A missense variant in the ATM gene (c.5429C > T; p.T1810I) was detected in an HPV-associated adenocarcinoma with micropapillary pattern (case 6). Two genetic variants were detected in a gastric-type adenocarcinoma with micropapillary pattern (case 14), including a missense variant in TP53 (c.469G > T; p.V157F) and a missense variant in SMARCB1 (c.215C > A; p.T72K).
Discussion
Primary cervical serous carcinoma was initially described as a morphologically defined entity resembling serous carcinomas elsewhere in the female genital tract [16, 17]. Based on several such cases [17,18,19], patients at stage I presented more favorable prognosis after radical surgery, similar to patients with usual endocervical adenocarcinomas, while those at advanced stages of the disease generally had less favorable outcomes. In a study of 13 cases diagnosed as cervical serous carcinoma, a dichotomized distribution was observed with better prognosis in premenopausal patients and worse outcomes in postmenopausal patients; a tubal origin was suggested for the postmenopausal cases as most were WT1 positive and some had concurrent serous tubal intraepithelial carcinoma [20]. A study by Togami et al. [21] demonstrated the presence of HPV DNA in 4 out of 10 patients with cervical serous carcinoma. Owing to inconsistent associations with HPV and the absence of a unifying biomarker in these cases, the existence of so-called “cervical serous carcinoma” has been subject to debate [3,4,5, 10].
Applying the paradigm of an HPV-based classification, primary cervical adenocarcinomas with serous-like features are believed to comprise mostly of HPV-associated adenocarcinomas. The terms “serous-like carcinoma” and “micropapillary adenocarcinoma” have been used to describe such cases as morphological variants of HPV-associated endocervical adenocarcinomas [4, 6]. The IECC study initially included serous adenocarcinoma as a subtype of HPV-independent adenocarcinoma [2], but this was found to be extremely rare in subsequent analyses [5, 22]. The ideal approach to classifying a primary cervical carcinoma with serous-like features is unclear and it should be based on evidence addressing the relationship of these morphological patterns with currently recognized histotypes. To the best of our knowledge, this study represents the first investigation applying the current HPV-based classification schema to primary cervical adenocarcinomas displaying serous-like morphological features, which essentially provides comprehensive data for the accurate diagnosis and appropriate management of these tumors.
The cases analyzed in this study suggest that serous-like papillary and micropapillary features are not restricted to specific histotypes of cervical carcinomas and both morphological patterns can be seen in HPV-associated as well as HPV-independent tumors. With the help of immunohistochemical analysis and HPV testing, the cases in our study were classified as either of usual endocervical adenocarcinoma, adenosquamous carcinoma, or gastric-type adenocarcinoma and based on their pathological profiles, none of them was classified as serous carcinoma analogous to the endometrial or ovarian counterparts.
The clinical outcomes of cervical carcinomas with serous-like features are generally congruent with the expected prognostic divergence between HPV-associated and HPV-independent tumors. Among cases with adequate follow-up, the 5-year overall survival for the HPV-associated group was 75%, as compared to 0% for the HPV-independent group. Recognizing that 5 of 9 HPV-associated cases with follow-up developed recurrence, metastasis or tumor-related death, notwithstanding the small sample size that precluded conclusive statements, this might indicate that the disease-free survival for these cases could be worse than that of conventional HPV-associated tumors.
While the micropapillary pattern only recently gained attention in cervical carcinomas and mostly described in HPV-associated cases, this feature has been extensively studied in carcinomas of other sites [23], currently defining a specific histotype in the breast [24], lung [25], urinary tract [26], salivary gland [27], stomach [28], and colon [29, 30]. Studies of micropapillary carcinomas in these various locations have generally demonstrated these cases to be more aggressive than their non-micropapillary counterparts [24,25,26,27,28,29,30]. Similarly, the study by Alvarado-Cabrero et al. [10] found relatively aggressive behavior among cervical carcinomas with micropapillary components. It is worth noting that in our study, regardless of HPV status, most tumors with a micropapillary pattern showed circumferential involvement of the cervix upon clinical examination, in contrast to the often localized involvement for those with a papillary pattern. All tumors with a micropapillary pattern also had Silva pattern C of invasion. These suggest a micropapillary pattern signifies a more extensive disease although this was not reflected in the FIGO stage probably because of the small sample size.
Among the reported cases of cervical carcinomas with a micropapillary pattern, 2–50% of cases were associated with an adenosquamous carcinoma [9, 10]. In our study, we demonstrated that adenosquamous carcinomas could exhibit serous-like features, characterized by papillary pattern in one and micropapillary in the other. Even among those cases without overt squamous components, immunohistochemical expression of p40 was focally present in two HPV-associated adenocarcinomas, which might suggest focal squamous differentiation. Coexisting HSIL was also present in two HPV-associated adenocarcinomas (one of them focally expressing p40), which was similarly encountered with previous reports of cervical carcinomas with micropapillary features [7,8,9]. These observations might suggest a possible link with squamous differentiation in a subset of cervical carcinomas displaying serous-like features.
Cases of gastric-type adenocarcinoma in this study illustrated the broad morphological spectrum and occasional unexpected immunoprofile of this tumor. In a gastric-type adenocarcinoma with serous-like papillary pattern (case 11), the finding of block positive p16 staining could have been misdiagnosed as HPV-associated carcinoma. An awareness of this phenomenon of block-type p16 expression in 4.3–33% of gastric-type adenocarcinomas [31, 32], with additional sampling of the areas of gastric-type morphology and HPV testing would be needed to avoid misdiagnosis.
Studies have identified HER2 overexpression or amplification in a few cervical adenocarcinomas [31, 33] and its potential therapeutic utility has gained attention [34]. As HER2 overexpression is commonly encountered in uterine serous carcinoma [35] and micropapillary urothelial carcinoma [36], this might also be the case with cervical adenocarcinomas displaying serous-like papillary or micropapillary features. Despite the limited sample size, our findings indicate that HER2 overexpression is implicated in at least a subset of cervical carcinomas with serous-like features, as this was observed in two cases with serous-like papillary pattern (including one HPV-independent tumor) and one case with micropapillary component. Whether this represents a clinically relevant therapeutic target requires further evaluation in larger clinical studies.
With immune checkpoint inhibitors becoming popular treatment options for metastatic cervical cancer [37, 38], we also assessed the expression of PD-L1—the standard biomarker for patient selection [14]. The four cases with positive results for PD-L1 expression were all HPV-associated, possibly related to the production of virus-induced antigens in these tumors [37]. Our findings suggested that PD-L1 expression may be occasionally encountered in cervical carcinomas with serous-like features.
The missense variant in ATM (p.T1810I) identified in the micropapillary component of a HPV-associated adenocarcinoma (case 6) was likely pathogenic based on the FATHMM prediction score of 0.93 (cancer.sanger.ac.uk); this variant had also been reported in a case of gastric carcinoma [39]. In a recent study by Hodgson et al. [40], ATM mutations were noted in 2 cases of gastric-type adenocarcinoma but none of 45 HPV-associated adenocarcinomas. TP53 abnormalities are common in gastric-type adenocarcinomas [33, 40]. The missense TP53 variant (p.V157F), found in a case of gastric-type adenocarcinoma (case 14), had conflicting interpretations of pathogenicity by ClinVar, with a neutral FATHMM prediction score of 0.24 (cancer.sanger.ac.uk). Experimental studies had provided evidence for the downstream effects of this variant [41, 42], which, coupled with the mutation-type immunoreactivity, suggest that this variant is possibly pathogenic. This case also revealed a missense variant in SMARCB1, previously found in a case of endometrial gastric (gastrointestinal)-type adenocarcinoma [43]. Although the significance of this variant is unclear, it may warrant further studies to establish an association with gastric-type gynecologic lesions.
The differential diagnosis of cervical carcinomas with serous-like morphological features can be challenging. Serous carcinomas of endometrial and tubo-ovarian origins may have substantial cervical involvement or an “in-situ” growth pattern mimicking a cervical primary [44] and these cases may have papillary and/or micropapillary components; this potentially accounts for certain cases previously reported as being “cervical serous carcinoma” [4, 6]. A few cases were excluded from this study based on the concurrent finding of serous carcinoma (including serous endometrial intraepithelial carcinoma) in the endometrium. WT1 immunohistochemistry would be helpful for those of tubo-ovarian primary, although 34–64% of endometrial serous carcinomas may also be WT1 positive [45, 46]. Conversely, both HPV-associated and HPV-independent cervical adenocarcinomas may also have extensive endometrial involvement [47, 48], reiterating that the predominant site of involvement does not reliably indicate the site of origin. It should be noted that both p16 and p53 have limitations in this scenario, as endometrial serous carcinoma can have diffuse positivity for p16 and cervical adenocarcinomas can have mutation-type p53 expression [31]. For HPV-associated adenocarcinomas (or adenosquamous carcinomas) with serous-like features, HPV testing is useful to confirm diagnosis. For gastric-type adenocarcinomas with serous-like foci, the coexisting component of typical gastric-type morphology and expression of gastrointestinal markers would be beneficial.
While the HPV-independent tumors in this study were of gastric-type, other uncommon subtypes of cervical carcinoma could also display serous-like features. Cervical mesonephric carcinoma can display papillary architecture, but these tumors often show characteristic oval, pale nuclei rather than high-grade nuclear atypia [6, 49]. The presence of associated mesonephric hyperplasia and the expression of GATA3 serve as diagnostic aids. Clear cell carcinomas may also harbor serous-like papillary structures, but the other typical morphological features and the expression of Napsin A are beneficial in diagnosis. Thus far, to our knowledge, micropapillary pattern has not been described in mesonephric or clear cell carcinoma.
Morphological suspicion of micropapillary component may be encountered with cervical carcinomas of diverse histologies. We have excluded 6 cases from our study based on the lack of typical “inside-out” immunoreactivity with EMA and MUC1, despite the initial impression of a micropapillary component. Upon reviewing the micropapillary-like foci, most of these cases were regarded as tumor budding, retraction artifacts, or lymphovascular invasion. Given its significance, we recommend using immunohistochemistry when confronted with a papillary/micropapillary carcinoma in the cervix.
The retrospective nature of the study was an inherent limitation because treatment modalities have changed and evolved over the study period. With four cases being biopsies, it might be arguable whether a tubo-ovarian or endometrial primary could be conclusively excluded even with imaging and endometrial sampling, and for those with bilateral salpingo-oophorectomy performed, the fallopian tubes were not processed using the SEE-FIM protocol. Due to the small sample size, we were unable to draw concrete conclusions on prognosis, but we believe that by applying strict criteria in the case selection, the observations in our study were noteworthy.
In summary, our study has demonstrated that primary endocervical adenocarcinomas can mimic the appearance of serous carcinoma, while not representing serous carcinoma. The results of our study indicate that cervical carcinomas with serous-like morphology can be re-classified as one of the histotypes recognized by the IECC or the upcoming 2020 WHO classification, basically either HPV-associated or gastric-type in our cohort. We believe these findings have provided the evidence in support of excluding serous carcinoma from the list of cervical adenocarcinoma subtypes. We propose that the presence of serous-like papillary and/or micropapillary patterns in cervical carcinomas may be documented as morphological patterns along with the HPV status and histologic subtype, to allow further studies on this subset of tumors.
References
Kurman RJ, Carcangiu ML, Herrington CS, Young RH, editors. WHO classification of tumours of female reproductive organs. Lyon: International Agency for Research on Cancer; 2014.
Stolnicu S, Barsan I, Hoang L, Patel P, Terinte C, Pesci A, et al. International endocervical adenocarcinoma criteria and classification (IECC): a new pathogenetic classification for invasive adenocarcinomas of the endocervix. Am J Surg Pathol. 2018;42:214–26.
Hodgson A, Park KJ, Djordjevic B, Howitt BE, Nucci MR, Oliva E, et al. International endocervical adenocarcinoma criteria and classification: validation and interobserver reproducibility. Am J Surg Pathol. 2019;43:75–83.
Park KJ. Cervical adenocarcinoma: integration of HPV status, pattern of invasion, morphology and molecular markers into classification. Histopathology. 2020;76:112–27.
Hodgson A, Olkhov-Mitsel E, Howitt BE, Nucci MR, Parra-Herran C. International endocervical adenocarcinoma criteria and classification (IECC): correlation with adverse clinicopathological features and patient outcome. J Clin Pathol. 2019;72:347–53.
Hodgson A, Park KJ. Cervical adenocarcinomas: a heterogeneous group of tumors with variable etiologies and clinical outcomes. Arch Pathol Lab Med. 2019;143:34–46.
Toyoda S, Kita T, Sugiura A, Itani Y, Okada H, Nakamura S, et al. Cervical adenocarcinoma with stromal micropapillary pattern. Diagn Cytopathol. 2016;44:133–6.
Munakata S, Hosoi A, Yamamoto T. Invasive micropapillary carcinoma of the uterine cervix: case report of a rare entity. Int J Gynecol Pathol. 2018;37:368–71.
Stewart CJR, Koay MHE, Leslie C, Acott N, Leung YC. Cervical carcinomas with a micropapillary component: a clinicopathological study of eight cases. Histopathology. 2018;72:626–33.
Alvarado-Cabrero I, McCluggage WG, Estevez-Castro R, Pérez-Montiel D, Stolnicu S, Ganesan R, et al. Micropapillary cervical adenocarcinoma: a clinicopathologic study of 44 cases. Am J Surg Pathol. 2019;43:802–9.
Pirog EC, Park KJ, Kiyokawa T, Zhang X, Chen W, Jenkins D, et al. Gastric-type adenocarcinoma of the cervix: tumor with wide range of histologic appearances. Adv Anat Pathol. 2019;26:1–12.
Köbel M, Ronnett BM, Singh N, Soslow RA, Gilks CB, McCluggage WG. Interpretation of P53 immunohistochemistry in endometrial carcinomas: toward increased reproducibility. Int J Gynecol Pathol. 2019;38:S123–31.
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human epidermal growth factor receptor 2 testing in breast cancer: american society of clinical oncology/college of american pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36:2105–22.
Ngoi NYL, Heong V, Lee XW, Huang YQ, Thian YL, Choo BA, et al. Tumor molecular profiling of responders and non-responders following pembrolizumab monotherapy in chemotherapy resistant advanced cervical cancer. Gynecol Oncol Rep. 2018;24:1–5.
de Leng WW, Gadellaa-van Hooijdonk CG, Barendregt-Smouter FA, Koudijs MJ, Nijman I, Hinrichs JW, et al. Targeted next generation sequencing as a reliable diagnostic assay for the detection of somatic mutations in tumours using minimal DNA amounts from formalin fixed paraffin embedded material. PLoS ONE. 2016;11:e0149405.
Gilks CB, Clement PB. Papillary serous adenocarcinoma of the uterine cervix: a report of three cases. Mod Pathol. 1992;5:426–31.
Zhou C, Gilks CB, Hayes M, Clement PB. Papillary serous carcinoma of the uterine cervix: a clinicopathologic study of 17 cases. Am J Surg Pathol. 1998;22:113–20.
Nofech-Mozes S, Rasty G, Ismiil N, Covens A, Khalifa MA. Immunohistochemical characterization of endocervical papillary serous carcinoma. Int J Gynecol Cancer. 2006;16(Suppl 1):286–92.
Togami S, Kasamatsu T, Sasajima Y, Onda T, Ishikawa M, Ikeda S, et al. Serous adenocarcinoma of the uterine cervix: a clinicopathological study of 12 cases and a review of the literature. Gynecol Obstet Investig. 2012;73:26–31.
Domfeh AB, Kuhn E, Park K, Parkash V. Papillary serous carcinoma of the cervix – two diseases with distinct clinico-pathologic profiles? Mod Pathol. 2013;26:272A.
Togami S, Sasajima Y, Kasamatsu T, Oda-Otomo R, Okada S, Ishikawa M, et al. Immunophenotype and human papillomavirus status of serous adenocarcinoma of the uterine cervix. Pathol Oncol Res. 2015;21:487–94.
Stolnicu S, Hoang L, Chiu D, Hanko-Bauer O, Terinte C, Pesci A, et al. Clinical outcomes of HPV-associated and unassociated endocervical adenocarcinomas categorized by the international endocervical adenocarcinoma criteria and classification (IECC). Am J Surg Pathol. 2019;43:466–74.
Nassar H. Carcinomas with micropapillary morphology: clinical significance and current concepts. Adv Anat Pathol. 2004;11:297–303.
Marchiò C, Iravani M, Natrajan R, Lambros MB, Savage K, Tamber N, et al. Genomic and immunophenotypical characterization of pure micropapillary carcinomas of the breast. J Pathol. 2008;215:398–410.
Kamiya K, Hayashi Y, Douguchi J, Hashiguchi A, Yamada T, Izumi Y, et al. Histopathological features and prognostic significance of the micropapillary pattern in lung adenocarcinoma. Mod Pathol. 2008;21:992–1001.
Lopez-Beltran A, Montironi R, Blanca A, Cheng L. Invasive micropapillary urothelial carcinoma of the bladder. Hum Pathol. 2010;41:1159–64.
Nagao T, Gaffey TA, Visscher DW, Kay PA, Minato H, Serizawa H, et al. Invasive micropapillary salivary duct carcinoma: a distinct histologic variant with biologic significance. Am J Surg Pathol. 2004;28:319–26.
Eom DW, Kang GH, Han SH, Cheon GJ, Han KH, Oh HS, et al. Gastric micropapillary carcinoma: a distinct subtype with a significantly worse prognosis in TNM stages I and II. Am J Surg Pathol. 2011;35:84–91.
Verdú M, Román R, Calvo M, Rodón N, García B, González M, et al. Clinicopathological and molecular characterization of colorectal micropapillary carcinoma. Mod Pathol. 2011;24:729–38.
Lee HJ, Eom DW, Kang GH, Han SH, Cheon GJ, Oh HS, et al. Colorectal micropapillary carcinomas are associated with poor prognosis and enriched in markers of stem cells. Mod Pathol. 2013;26:1123–31.
Stolnicu S, Barsan I, Hoang L, Patel P, Chiriboga L, Terinte C, et al. Diagnostic algorithmic proposal based on comprehensive immunohistochemical evaluation of 297 invasive endocervical adenocarcinomas. Am J Surg Pathol. 2018;42:989–1000.
Carleton C, Hoang L, Sah S, Kiyokawa T, Karamurzin YS, Talia KL. et al. A detailed immunohistochemical analysis of a large series of cervical and vaginal gastric-type adenocarcinomas. Am J Surg Pathol. 2016;40:636–44.
Garg S, Nagaria TS, Clarke B, Freedman O, Khan Z, Schwock J, et al. Molecular characterization of gastric-type endocervical adenocarcinoma using next-generation sequencing. Mod Pathol. 2019;32:1823–33.
Oh DY, Kim S, Choi YL, Cho YJ, Oh E, Choi JJ, et al. HER2 as a novel therapeutic target for cervical cancer. Oncotarget. 2015;6:36219–30.
Fader AN, Roque DM, Siegel E, Buza N, Hui P, Abdelghany O, et al. Randomized Phase II trial of carboplatin-paclitaxel versus carboplatin-paclitaxel-trastuzumab in uterine serous carcinomas that overexpress human epidermal growth factor receptor 2/neu. J Clin Oncol. 2018;36:2044–51.
Sanguedolce F, Russo D, Mancini V, Selvaggio O, Calo B, Carrieri G, et al. Prognostic and therapeutic role of HER2 expression in micropapillary carcinoma of the bladder. Mol Clin Oncol. 2019;10:205–13.
Borcoman E, Le Tourneau C. Pembrolizumab in cervical cancer: latest evidence and clinical usefulness. Ther Adv Med Oncol. 2017;9:431–9.
Liu Y, Wu L, Tong R, Yang F, Yin L, Li M, et al. PD-1/PD-L1 inhibitors in cervical cancer. Front Pharmacol. 2019;10:65.
Ito T, Matoba R, Maekawa H, Sakurada M, Kushida T, Orita H, et al. Detection of gene mutations in gastric cancer tissues using a commercial sequencing panel. Mol Clin Oncol. 2019;11:455–60.
Hodgson A, Howitt BE, Park KJ, Lindeman N, Nucci MR, Parra-Herran C. Genomic Characterization of HPV-related and gastric-type endocervical adenocarcinoma: correlation with subtype and clinical behavior. Int J Gynecol Pathol. 2019. https://doi.org/10.1097/PGP.0000000000000665.
Calhoun S, Daggett V. Structural effects of the L145Q, V157F, and R282W cancer-associated mutations in the p53 DNA-binding core domain. Biochemistry. 2011;50:5345–53.
Barta JA, Pauley K, Kossenkov AV, McMahon SB. The lung-enriched p53 mutants V157F and R158L/P regulate a gain of function transcriptome in lung cancer. Carcinogenesis. 2020;41:67–77.
Wong RW, Ralte A, Grondin K, Talia KL, McCluggage WG. Endometrial gastric (gastrointestinal)-type mucinous lesions: report of a series illustrating the spectrum of Benign and Malignant Lesions. Am J Surg Pathol. 2020;44:406–19.
McCluggage WG, Hurrell DP, Kennedy K. Metastatic carcinomas in the cervix mimicking primary cervical adenocarcinoma and adenocarcinoma in situ: report of a series of cases. Am J Surg Pathol. 2010;34:735–41.
Zhang Y, Garcia-Buitrago MT, Koru-Sengul T, Schuman S, Ganjei-Azar P. An immunohistochemical panel to distinguish ovarian from uterine serous papillary carcinomas. Int J Gynecol Pathol. 2013;32:476–81.
Chen W, Husain A, Nelson GS, Rambau PF, Liu S, Lee CH, et al. Immunohistochemical profiling of endometrial serous carcinoma. Int J Gynecol Pathol. 2017;36:128–39.
Yemelyanova A, Vang R, Seidman JD, Gravitt PE, Ronnett BM. Endocervical adenocarcinomas with prominent endometrial or endomyometrial involvement simulating primary endometrial carcinomas: utility of HPV DNA detection and immunohistochemical expression of p16 and hormone receptors to confirm the cervical origin of the corpus tumor. Am J Surg Pathol. 2009;33:914–24.
Kamiya A, Ikura Y, Iizuka N, Yokokawa T, Kato H, Oishi T. Gastric-type endocervical adenocarcinoma with uterine corpus involvement mimicking primary endometrial carcinoma. J Obstet Gynaecol Res. 2019;45:1414–7.
Howitt BE, Nucci MR. Mesonephric proliferations of the female genital tract. Pathology. 2018;50:141–50.
Acknowledgements
We would like to thank the staff of the Histopathology Laboratory, Pamela Youde Nethersole Eastern Hospital, for performing the immunohistochemistry. We would like to thank Pui Yung Dorothy Chan and other staff of the Molecular Laboratory, Pamela Youde Nethersole Eastern Hospital, for performing the molecular studies. We would like to thank Dr Chun Kei Cheung for assistance with the molecular studies and Dr Ivy Wong for advice on data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wong, R.WC., Ng, J.H.Y., Han, K.C. et al. Cervical carcinomas with serous-like papillary and micropapillary components: illustrating the heterogeneity of primary cervical carcinomas. Mod Pathol 34, 207–221 (2021). https://doi.org/10.1038/s41379-020-0627-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-020-0627-8