Abstract
Nippostrongylus brasiliensis infections generate pulmonary pathologies that can be associated with strong TH2 polarization of the host's immune response. We present data demonstrating N. brasiliensis-driven airway mucus production to be dependent on smooth muscle cell interleukin 4 receptor-α (IL-4Rα) responsiveness. At days 7 and 10 post infection (PI), significant airway mucus production was found in IL-4Rα−/lox control mice, whereas global knockout (IL-4Rα−/−) and smooth muscle-specific IL-4Rα-deficient mice (SM-MHCCre IL-4Rα−/lox) showed reduced airway mucus responses. Furthermore, interleukin (IL)-13 and IL-5 cytokine production in SM-MHCCre IL-4Rα−/lox mice was impaired along with a transient reduction in T-cell numbers in the lung. In vitro treatment of smooth muscle cells with secreted N. brasiliensis excretory–secretory antigen (NES) induced IL-6 production. Decreased protein kinase C (PKC)-dependent smooth muscle cell proliferation associated with cell cycle arrest was found in cells stimulated with NES. Together, these data demonstrate that both IL-4Rα and NES-driven responses by smooth muscle cells make important contributions in initiating TH2 responses against N. brasiliensis infections.
Similar content being viewed by others
Introduction
Smooth muscle cells are implicated in the pathology of a number of diseases, in particular those associated with airway and intestinal hyperresponsiveness in allergy and parasitic nematode infections. Interleukin 4 receptor-α (IL-4Rα), the common component of the heterodimeric IL-4 and IL-13 receptors, also has key roles in these diseases.1, 2, 3, 4 IL-4Rα-dependent TH2 immune responses drive allergic airway pathology and are essential for the expulsion of intestinal parasitic nematodes.5 Use of mouse models deficient in TH2 signaling proteins such as IL-13,6 IL-4Rα,7 and STAT-6 (signal transducer and activator of transcription 6)8 have demonstrated the onset of experimental asthma9, 10 and host ability to expel intestinal nematodes11 to be highly impaired. Given the pleiotropic nature of the biology of IL-4/IL-13, more detailed and refined studies into the role of IL-13, IL-4Rα, and STAT-6 are likely to have important implications in our understanding of this signaling system. Recent studies using mice with cell-specific disruption of IL-4Rα expression have begun to unravel specific cellular roles for IL-4Rα in a number of pathologies.12, 13, 14, 15, 16, 17 We recently demonstrated in mice deficient in smooth muscle IL-4Rα expression (SM-MHCCre IL-4Rα−/lox) a delayed ability to expel the intestinal parasitic nematode Nippostrongylus brasiliensis.17 This effect related to delayed goblet cell hyperplasia, M3 muscarinic receptor expression, and IL-13 production in the intestine,18 concomitant with reduced intestinal contraction at 10 days post infection (PI).18 Furthermore, mesenteric lymph node-derived CD4+ T cells demonstrated sustained reductions in TH2 cytokine production. This unexpected observation suggested that smooth muscle cell IL-4Rα responsiveness may be required for efficient immune signaling from the intestinal lumen to the draining lymph nodes, and the stimulation of CD4+ T-cell TH2 cytokine secretions in the intestine. Whether this phenotype is also a feature of N. brasiliensis-induced pulmonary pathology or is limited to the intestinal tract has not been demonstrated.
N. brasiliensis larval migration from the circulatory to bronchial system causes direct damage to the lung during the first 48 h of infection.19 This damage to the pulmonary architecture is also accompanied by the development of parasite-induced pathology that shares a number of characteristics with allergic pathologies.20, 21
In this study we analyzed host responses to N. brasiliensis infection at days 3, 7, and 10 PI. At day 3 PI, parasites are completing their migration and moulting in the lung, and therefore this represents a time point for potential priming events during the onset of TH2 immunity. At day 7 PI, adult worms are established in the intestine and strong TH2-associated immunity is apparent. Day 10 PI is approximately 24 h post expulsion (in BALB/c IL-4Rα−/lox mice) and represents a time point when TH2 immune markers can be reduced in IL-4Rα−/lox mice. In this study we found N. brasiliensis-induced pulmonary pathology to be IL-4Rα dependent, with IL-4Rα responsiveness by smooth muscle cells contributing significantly to this type-2-mediated pathology, as measured by pulmonary infiltration by T cells and goblet cell hyperplasia. Moreover, levels of TH2 cytokines were significantly reduced in the mediastinal lymph nodes and lungs of SM-MHCCre IL-4Rα−/lox mice. In vitro studies of smooth muscle cell responses to secreted N. brasileinsis excretory–secretory antigen (NES) indicated strong parasite-associated induction of IL-6 and inhibition of cell proliferation. These data demonstrate important roles for smooth muscle cells in the onset and development of TH2 immune responses to N. brasiliensis.
Results
Reduced IL-4Rα exon 8 copy numbers in SM-MHCCre IL-4Rα−/lox pulmonary smooth muscle-enriched cell preparations
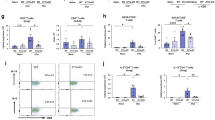
Smooth muscle cell-specific impairment of IL-4Rα expression was driven by Cre recombinase expression under the control of the smooth muscle myosin heavy chain (SM-MHC) promoter. This results in the excision, by Cre, of the loxP-flanked exons 7–9 of IL-4Rα DNA being confined to smooth muscle cells (Figure 1ai). All other cells contain a single functional allele for IL-4Rα (Figure 1aii). Single-cell suspensions of lung cells derived from naive control IL-4Rα−/lox and SM-MHCCre IL-4Rα−/lox mice showed equivalent numbers of α-actin-positive cells (Figure 1b) in both groups. Cell sorting resulted in an approximate 20-fold increase in the concentration of α-actin-positive cells in both control IL-4Rα −/lox and SM-MHCCre IL-4Rα−/lox mice (Figure 1b). Flow cytometric analysis failed to identify IL-4Rα on α-actin-positive cells derived from the lungs of either control IL-4Rα−/lox or SM-MHCCre IL-4Rα−/lox naive mice (data not shown). However, comparison of ratios of the excised Il4ra exon 8 with Il4ra exon 5 showed significant reductions in the ratio of exon 8 andexon 5 (Figure 1ci), because of efficient smooth muscle cell-specific deletion of IL-4Rα in the lung of SM-MHCCre IL-4Rα−/lox , whereas CD4+ T cells isolated from control IL-4Rα−/lox and SM-MHCCre IL-4Rα−/lox mice showed equivalent levels of expression (Figure 1cii). These results further support the specificity of the IL-4Rα disruption on smooth muscle cells in SM-MHCCre IL-4Rα−/lox mice. In agreement with this result and previously published data,22 flow cytometric analysis of IL-4Rα expression on immune cells isolated from naive lungs demonstrated IL-4Rα expression to be maintained on CD4+ T cells and CD19+ B cells (Figure 1d).
Reduced interleukin 4 receptor-α (IL-4Rα) exon 8 copy numbers in smooth muscle myosin heavy chain (SM-MHC)Cre IL-4Rα−/lox pulmonary smooth muscle-enriched cell preparations. (a) Schematic representation of Cre/LoxP-mediated disruption of IL-4Rα expression in smooth muscle cells. (i) SMC MHC specific Cre expression distrupts IL-4Rα expression on both alleles in smooth muscle cells. (ii) no Cre expression in non-smooth muscle cells does not disrupt remaining IL-4Ra gene in hemizygous control animals. (b) Single-cell suspension of lung cells derived from naive control IL-4Rα−/lox and SM-MHCCre IL-4Rα−/lox mice were stained for smooth muscle cell α-actin. α-Actin-enriched preparations were then prepared by cell sorting. FL-1: α-actin positive. (c) DNA expression ratios. Comparison of ratios of Il4ra exon 8 and Il4ra exon 5 were established in both smooth muscle cell-enriched preparations (i) and CD-4+ T cells (ii) isolated from control IL-4Rα−/lox and SM-MHCCre IL-4Rα−/lox mice. Data are representative of four individual mice per group. (d) Flow cytometric analysis of IL-4Rα expression on immune cells isolated from naive lungs demonstrates IL-4Rα expression to be maintained on CD4+ T cells, CD8+ T cells, and CD19+ B cells. Data are representative of a single experiment.
Airway mucus production is decreased in SM-MHCCre IL-4Rα−/lox mice
To establish if any differences in lung pathology following N. brasiliensis infection were because of IL-4Rα-dependent effects on the numbers of larvae migrating through the lung, and therefore parasite antigen deposition, or lung damage, we assessed L4 lung burdens at days 1 and 3 PI in IL-4Rα−/lox, IL-4Rα−/−, and SM-MHCCre IL-4Rα−/lox mice. No differences in worm burdens between transgenic mouse strains at days 1 and 3 PI (Figure 2a) were found.
Decreased airway mucus production in N. brasiliensis-infected smooth muscle myosin heavy chain (SM-MHC)Cre IL-4Rα−/lox mice. (a) Larval lung counts at days 1 and 3 post infection (PI). 4–6 mice were used per time point for each group. (b) Airway mucus production was visualized using periodic acid Schiff (PAS) reagent staining in naive mice and mice at days 3, 7, and 10 PI. Areas demonstrating positive PAS staining are enclosed by dashed white line. All images, original magnification × 200. (c) Histological mucus index (HMI) quantification of PAS-stained lung sections. Data are representative of three independent experiments. 4–6 mice were used per time point for each group. *P<0.05, significant difference from IL-4Rα−/lox mice. ND, not detected.
N. brasiliensis infections are characterized by induction of parasite-induced airway pathology. Examination and quantification of this response by periodic acid Schiff staining and calculation of histological mucus index (Figure 2c) in naive mice and infected mice at days 3, 7, and 10 PI demonstrated strong airway mucus responses in infected IL-4Rα −/lox mice at days 7 and 10 PI (Figure 2b, left panel and Figure 2c). IL-4Rα−/− mice failed to elicit an airway mucus response (Figure 2b, middle panels and Figure 2c). Although airway mucus responses were apparent in SM-MHCCre IL-4Rα−/lox mice at days 7 and 10 PI (Figure 2b, right panels), histological mucus index analysis demonstrated this to be significantly lower than that found in IL-4Rα−/lox mice (Figure 2b, left panels). These data demonstrate the requirement for IL-4Rα-responsive smooth muscle cells for airway mucus production induced during N. brasiliensis infection.
Disrupted T-cell recruitment in SM-MHCCre IL-4Rα−/lox mice
In order to identify potential immune cell populations driving airway mucus production, we examined eosinophil, mast cell, and T-cell populations in naive mice and mice at days 3, 7, and 10 PI (Figure 3). Examination of eosinophil numbers in the lung showed a reduction in IL-4Rα−/− mice when compared with control IL-4Rα −/lox at both days 7 and 10 PI. However, no effect was seen in the numbers of eosinophils in the lungs of SM-MHCCre IL-4Rα−/lox mice when compared with control IL-4Rα−/lox mice (Figure 3a). Mast cell numbers in the lung peaked at day 7 PI and decreased by day 10 PI, with no IL-4Rα-dependent differences in mast cell numbers found (Figure 3b).
Effect of disrupted smooth muscle cell interleukin 4 receptor-α (IL-4Rα) expression on pulmonary immune cell populations in N. brasiliensis-infected mice. Single-cell suspensions of whole lung from individual mice were analyzed by flow cytometry for numbers of (a) eosinophils (CD11c-low, GR-1-intermediate), (b) Mast cells (CD117+, FCɛR+), (c) CD3+, and (d) CD4+ cells present in the indicated mouse strains. Black bars represent control IL-4Rα−/lox mice, white IL-4Rα−/−, and striped smooth muscle myosin heavy chain (SM-MHC)Cre IL-4Rα−/lox mice. *Significant differences from control mice (P<0.05), 4 to 5 mice per group, data representative of two to three individual experiments.
As increased T-cell numbers in the lung have been implicated in the onset of allergic airway pathology,23 we examined if the absence of IL-4Rα on smooth muscle cells affects T-cell populations in the lungs of parasite-infected animals (Figure 3c, d). In naive and day 3 PI animals, T-cell numbers were either not detectable or extremely low in all mouse groups. Quantification of CD3+ (Figure 3c) and CD4+ (Figure 3d) T-cell populations demonstrated significantly lower numbers of both T-cell populations in IL-4Rα−/− and SM-MHCCre IL-4Rα−/lox mice when compared with control IL-4Rα−/lox mice at day 7 PI. At day 10 PI, significantly reduced numbers of CD3+ and CD4+ cells were still found in IL-4Rα−/− mice, whereas control IL-4Rα−/lox and SM-MHCCre IL-4Rα−/lox mice demonstrated equivalent populations of both CD3+ and CD4+ cells.
These results show an association between reduced airway mucus production seen in SM-MHCCre IL-4Rα−/lox mice and transient reduction in T-cell numbers in the infected lung.
Impaired MST lymph node TH2 cytokine responses in SM-MHCCre IL-4Rα−/lox mice
TH2 cytokine production by T cells correlates strongly with the onset of pulmonary allergic pathology.24 In particular, the TH2 cytokines IL-5 and IL-13 have been associated with the generation of parasite-induced pathology. In order to establish if the disrupted onset of N. brasiliensis-induced pulmonary pathology in SM-MHCCre IL-4Rα−/lox mice was also because of an impaired TH2 cytokine response, we restimulated CD4+ T cells isolated from the mediastinal lymph nodes (MST) of N. brasiliensis-infected mice at days 7 and 10 PI with the mitogen anti-CD3 (Figure 4). Restimulated CD4+ T cells from both IL-4Rα−/− and SM-MHCCre IL-4Rα−/lox mice demonstrated significantly lower IL-5 and IL-13 TH2 cytokine production when compared with control IL-4Rα −/lox mice. The TH1 cytokine interferon-γ was elevated in IL-4Rα−/− mice at day 7 PI. No significant differences were found in interferon-γ levels between IL-4Rα−/lox and SM-MHCCre IL-4Rα−/lox mice. These results are in agreement with previously published data from the mesenteric lymph nodes of N. brasiliensis-infected mice,17 and are indicative that IL-4Rα-responsive smooth muscle cells may influence CD4+ T-cell cytokine secretion during N. brasiliensis infections in the draining lymph node.
Disrupted smooth muscle interleukin 4 receptor-α (IL-4Rα) expression results in reduced TH2 cytokine secretion. CD4+ lymphocytes isolated from MST from infected mice on days 3, 7, and 10 post infection (PI) were restimulated with anti-CD3 for 72 h. Supernatants were then analyzed for cytokine (a) IL-13, (b) IL-5, and (c) interferon-γ (IFN-γ) by enzyme-linked immunosorbent assay (ELISA). *P<0.05, significant difference from IL-4Rα−/lox mice. In all, 4–6 mice were used per time point for each group. Black bars represent control IL-4Rα−/lox mice, white IL-4Rα−/−, and striped smooth muscle myosin heavy chain (SM-MHC)Cre IL-4Rα−/lox mice. Data are representative of three independent experiments. ND, not detected.
The significant reduction in eosinophils in IL-4Rα−/− mice at days 7 and 10 PI (Figure 3a) can be explained by reduced IL-5 production from anti-CD3 restimulated MST CD4+ from IL-4Rα−/− mice. The observed slight but significant decrease in IL-5 levels from day 10 infected SM-MHCcreIL-4Rα−/− mice did not appear to be sufficient to reduce eosinophilia at this time point.
In summary, these cytokine data further highlight the influence of IL-4Rα-responsive smooth muscle cells on the differentiation and subsequent TH2 cytokine response during N. brasiliensis infection.
Disrupted cytokine and chemokine responses in the lungs of SM-MHCCre IL-4Rα−/lox mice
In order to further assess the impact of local immune responses, we also analyzed cytokine and chemokine concentrations directly from homogenates of lungs from N. brasiliensis-infected mice (Figure 5).
Effect of smooth muscle interleukin 4 receptor-α (IL-4Rα) disruption on lung cytokine and chemokine levels. Whole lungs were removed from infected mice at days 3, 7, and 10 post infection (PI) and homogenized in lysis buffer containing protease inhibitors. Protein concentration of the homogenates were quantified using the bicinchoninic acid (BCA) assay and adjusted to 5 mg ml–1 after which the levels of the cytokines (a) IL-13 and chemokines (b) macrophage inflammatory protein-1α (MIP-1α) and (c) eotaxin were analyzed using enzyme-linked immunosorbent assay (ELISA). Four mice of each strain were used at every time point, and data are representative of two independent experiments. Significant differences are represented as *P<0.05 and ***P<0.001.
Interestingly, IL-13 levels were substantially reduced at days 7 and 10 PI in IL-4Rα−/lox mice in both IL-4Rα−/− and SM-MHCCre IL-4Rα−/lox mice when compared with IL-4Rα−/lox (Figure 5a).22 We also examined the levels of the chemokines macrophage inflammatory protein-1α (MIP-1α) and eotaxin in the lung (Figure 5b,c). MIP-1α levels in the lung were significantly elevated in IL-4Rα−/lox mice at day 10 PI when compared with IL-4Rα−/− and SM-MHCCre IL-4Rα−/lox mice, which is in agreement with reports of MIP-1α being related to resistance to helminth infections.25, 26 Eotaxin levels were in agreement with our observations of eosinophil populations in the lung; namely, eotaxin levels in the lung were reduced in IL-4Rα−/− mice when compared with control IL-4Rα−/lox at both days 7 and 10 PI. However, no effect was seen in eotaxin levels in SM-MHCCre IL-4Rα−/lox mice when compared with control IL-4Rα−/lox mice (Figure 5c). Together, these results suggest that smooth muscle-specific IL-4Rα responsiveness influences not only TH2 cytokine responses but also MIP-1α.
N. brasiliensis antigen effects on smooth muscle cell responses
Having established that IL-4Rα expression on smooth muscle cells is required for optimal TH2 immunity to N. brasiliensis, we investigated to what extent the direct interaction between N. brasiliensis and smooth muscle cells may influence this response. Specifically, we examined in vitro how secreted NES affected smooth muscle cell cytokine responses, IL-4Rα surface expression, and smooth muscle cell proliferation.
Following incubation of smooth muscle cells with NES, we found significantly higher IL-6 mRNA expression (Figure 6a) and protein secretion (data not shown) when compared with phosphate-buffered saline (PBS)-treated controls. Surface expression of IL-4Rα did not appear to be affected following exposure to NES (Figure 6b).
Smooth muscle cell immune and proliferative response to N. brasiliensis excretory–secretory antigen (NES). (a) IL-6 production by smooth muscle cells following incubation with NES was measured by reverse transcriptase-PCR (RT-PCR). All RT-PCR data were normalized against rs12 expression. (b) Interleukin 4 receptor-α (IL-4Rα) expression by smooth muscle cells following incubation with NES was measured by flow cytometry and represented as (i) histogram from one of each treatment and (ii) mean fluorescence intensity of IL-4Rα from three replicates of each treatment. (c) (i) NES inhibits smooth muscle cell proliferation in a dose-dependent manner. (ii) Inhibition of smooth muscle cell proliferation is related to arrest of cell cycle progression by NES. (d) NES inhibits protein kinase C (PKC)-induced smooth muscle cell proliferation. All data are representative of two to three similar experiments. Significant differences: *P<0.05, **P<0.01, and ***P<0.001.
Interestingly, NES treatment of smooth muscle cells resulted in a profound inhibition of smooth muscle cell proliferation (Figure 6ci). Furthermore, we demonstrated that NES-treated cells were arrested at the S phase of the cell cycle (Figure 6cii). Increased cellular proliferation following incubation with the protein kinase C (PKC) agonist phorbol myristate acetate was also inhibited by NES. NES-mediated inhibition of PKC-driven proliferation was equivalent to that seen following incubation with the PKC inhibitor bisindolymaleimide (Figure 6d). Together, these data suggest that smooth muscle cell proliferation may be inhibited by exposure to NES through disruption of PKC-dependent signaling events.
Discussion
In this study we have demonstrated that smooth muscle cell IL-4Rα-dependent signaling influences parasite-induced airway mucus production. Associated with this we found IL-4Rα-dependent increases in pulmonary T-cell populations and production of the cytokines IL-5 and IL-13 along with the chemokine MIP-1α. We also observed that smooth muscle cell IL-4Rα-dependent responses did not affect eosinophil or mast cell responses. Although both these cell types are implicated in the resolution of a number of helminth infections,27, 28, 29 their roles in N. brasiliensis immunity is limited.30 These results extend previous observations by us that in vivo IL-4Rα-responsive smooth muscle cells are beneficial for N. brasiliensis expulsion by coordinating TH2 cytokine responses, goblet cell hyperplasia, and M3 receptor expression in the intestine, all of which are potential mediators of worm expulsion.17 Together, with the current data, a more general role for smooth muscle cell IL-4Rα signaling in the onset of local immune responses and the development of TH2 responses following N. brasiliensis infection is apparent. The roles for smooth muscle cell IL-4Rα expression in other pulmonary pathologies, such as allergic airway hyperresponsiveness, have been demonstrated using in vitro studies.4, 31 However, ovalbumin-induced allergic asthma in SM-MHCCre IL-4Rα−/lox mice did not have an influence on airway hyperresponsiveness or goblet cell hyperplasia.32 This surprising difference from the study presented here indicates that in vivo N. brasiliensis-induced airway mucus production is initiated by alternative mechanisms to those found in ovalbumin-induced allergic asthma.
In order to define immunological events that could underlie the reduced airway mucus production in SM-MHCCre IL-4Rα−/lox mice, we examined the effects that this disrupted expression of IL-4Rα may have on immune cell populations associated with allergic-like lung pathologies. Requirements for IL-4/IL-13, IL-4Rα, and STAT-6 signaling in T-cell recruitment to the lung have been demonstrated in both ovalbumin-induced allergy33 and N. brasiliensis-associated lung pathology.34, 35, 36 The data that we present in this study indicate that at least in N. brasiliensis infections, expression of IL-4Rα on smooth muscle cells has an important role for T-cell recruitment to the lung. Moreover, expression of IL-4Rα on smooth muscle cells had a surprisingly strong effect on the hosts’ ability to generate a TH2 cytokine response. We suggest that the combination of both, reduced pulmonary T-cell populations and TH2 cytokines, may underlie observed reductions in parasite-induced pathology in SM-MHCCre IL-4Rα−/lox mice.
In addition to classical TH2 cytokine responses, we also found optimal production of the chemokine MIP-1α to be related to smooth muscle cell IL-4Rα expression. MIP-1α has also previously been shown to induce TH2 cytokine responses in the lung and intestine.37 Elevated MIP-1α levels have also been associated with protection against helminth infections,25 although non-hematopoietic cell contributions to MIP-1α production have not been previously demonstrated.38 As N. brasiliensis infections are resolved at day 9 PI, our observation of IL-4Rα-dependent increases in MIP-1α at day 10 PI may be indicative of a systemic increase in MIP-1α having a role in worm expulsion.
Having demonstrated IL-4Rα-expressing smooth muscle cells to be important in inducing TH2 immunity to N. brasiliensis in the lung, we examined whether direct interactions between the parasite and smooth muscle cells could also influence host immunity. Smooth muscle cells have been shown to be effective antigen-presenting cells39, 40, 41 capable of inducing strong TH2 T-cell responses.42, 43 In agreement with this we also found potential roles for parasite antigen directly modulating smooth muscle cell responses. Of particular interest was increased IL-6 production by smooth muscle cells in response to NES. IL-6 has been shown to be produced by smooth muscle cells,44, 45 and is associated with inhibiting TH1 and promoting TH2 immunity.46, 47 Elevated IL-6 production by smooth muscle cells in response to NES would also agree with other studies demonstrating IL-6 production by smooth muscle cells in response to exposure to TH2-associated antigens and cytokines.44
Additionally, we found that NES inhibited smooth muscle cell proliferation. This appears to be a feature of exposure to nematode antigens, which has previously been reported in lymphocytes.48, 49, 50 It has been suggested that this inhibition of host cell proliferation is an example of parasite-driven immune evasion. However, elevated IL-6 cytokine production in smooth muscle cells could also indicate this decreased proliferative response to be an example of a change in cellular commitment in order to control the parasitic infection.
In conclusion, this study demonstrates important roles for smooth muscle cell IL-4Rα in inducing pulmonary pathologies associated with N. brasiliensis infections. We also demonstrate that N. brasiliensis antigen has profound effects on smooth muscle cell biology that may explain, in part, the contribution of smooth muscle cell responses to TH2 immunity. We suggest that IL-4Rα-dependent smooth muscle cell production of cytokines, such as IL-6, may contribute to initial recruitment of immune cell populations to the lung. The resulting direct interactions between these cells and antigen-presenting smooth muscle cells may form an important component in the initiation of TH2 immunity to parasitic helminth infections.
Methods
Animals
Female 6- to 8-week-old IL-4Rα−/lox, IL-4Rα−/−,51and SM-MHCCre IL-4Rα−/lox mice17 on BALB/c backgrounds were used in all experiments. SM-MHCCre IL-4Rα−/lox mice were backcrossed to BALB/c for at least nine generations.17 Animals were housed in independently ventilated cages under specific pathogen-free conditions in the University of Cape Town Animal Facility. Animal procedures used in this study were approved by the animal ethics committee of the University of Cape Town (license no. 006/09).
Infection of mice with N. brasiliensis
Mice were injected subcutaneously with 750 N. brasiliensis L3 larvae (originally provided by Klaus Erb, Wurzburg, Germany). Analysis of parasite eggs in feces was carried out using the Modified McMaster technique.52 Adult worm burdens were determined as previously described.7
Larval lung counts
Quantification of L4 larval loads in the lung of infected mice was carried out at days 1 and 3 PI. Larval numbers were calculated as previously described.53
Preparation of smooth muscle cell-enriched lung preparations
PBS-perfused lungs were removed from euthanized mice. Lungs were finely cut and digested in Dulbecco's modied Eagle's medium (DMEM; Invitrogen, Carlsbad, CA) with 50 U ml–1 collagenase type I (Invitrogen), 13 μg ml–1 DNAse I (Roche, Basel, Switzerland), and 2 mM EDTA at 37 °C for 90 min. Samples were pushed through a 70-μm cell strainer and subjected to red blood cell lysis. Cells were then stained for the smooth muscle cell marker α-actin as previously described.22 Cells were then isolated using a FACSVantage cell sorter (BD Biosciences, San Jose, CA). Genomic DNA was isolated from both CD4+ lymphocytes and α-actin sorted lung cells of SM-MHCcreIL-4Rα−/lox and IL-4Rα−/lox mice. The copy numbers of IL-4Rα exon 5 and IL4-Rα exon 8 were determined using a Roche Lightcycler (Roche) and amplification was monitored using SYBR Green I Cloned IL-4Rα exon 5 and exon 8 DNA was used to prepare a standard curve. The primers were as follows: exon 5 forward 5′-AACCTGGGAAGTTGTG-3′, exon 5 reverse 5′-CACAGTTCCATCTGGTAT-3′; exon 8 forward 5′-GTACAGCGCACATTGTTTTT-3′ and exon 8 reverse 5′-CTCGGCGCACTGACCCATCT-3′.
Histology
Tissue samples were fixed in a neutral buffered formalin solution. Following embedding in paraffin, samples were cut into 5–7 μm sections. Sections were stained with periodic acid Schiff reagent. The histological mucus index was used to quantify pulmonary goblet cell hyperplasia in individual mice. Here, airways were photographed at × 100 magnification and overlaid with a standard grid. The number of mucus-positive epithelial cells were counted and expressed as a percentage of the total number of epithelial cells in order to determine histological mucus index. All samples were randomized and counted by a blinded observer.
PSMC-1 cell culture
Mouse prostatic smooth muscle cell (PSMC-1)54 cultures were maintained in DMEM medium supplemented with 10% endotoxin-free fetal calf serum (Invitrogen), 100 U ml–1 penicillin, and 100 μg ml–1 streptomycin in an incubator at 37 °C and 5% CO2.
Reverse transcriptase-PCR
RNA was extracted from PSMC-1 cells using Tri-reagent (Sigma, St Louis, MO) and complementary DNA was synthesized using the ImProm-II Reverse Transcription System (Promega, Madison, WI). Complementary DNA was amplified using the following primers: IL-4Rα: 5′-ACTGGATCTGGGAGCATCAA-3′ and 5′-CCTATTCATTTCCATGTGGCA-3′ IL-4: 5′-TCGGCATTTTGAACGAGGTC-3′ and 5′-GAAAAGCCCGAAAGAGTCTC-3′ IL-13: 5′-CTCACTGGCTCTGGGCTTCA-3′ and 5′-CTCATTAGAAGGGGCCGTGG-3′ IL-6: 5′-GTTCTCTGGGAAATCGTGGA-3′ and 5′-TGTACTCCAGGTAGCTATGG-3′. Data were normalized using β-actin or rs12 housekeeping genes.
Ex vivo restimulation of lymphocytes
CD4+ T cells were enriched from pooled mediastinal lymph nodes (MST) at days 7 and 10 PI. Enrichment was carried with a negative selection. Briefly, cells in single-cell suspension were stained with anti-CD8α (53-6.7), CD11b (M1/70), Gr-1 (RB6-8C5), and B220 (RA3-6B2). Stained cells were depleted using goat anti-rat IgG-coated magnetic beads (Biomag beads; Qiagen, Hilden, Germany). Cell preparations used consisted of ≥94% CD4+ cells. CD4+ T cells were plated out at 2 × 106 cells per ml and restimulated for 72 h with anti-CD3 (clone 145-2C11; 20 μg ml–1). Supernatants were then collected and stored at −80 °C until analysis.
Enzyme-linked immunosorbent assay analysis
Enriched CD4+ T-cell populations were plated out at 1 × 106 cells per ml on flat-bottomed 96-well plates coated with 20 μg ml–1 αCD3 or PBS. Following 72 h of incubation, cytokine levels in supernatants from restimulated CD4+ T cells were determined as previously described.55 For lung cytokine and chemokine detection, whole lung was removed from infected mice and homogenized in lysis buffer containing protease inhibitors (Sigma). The homogenates were centrifuged at 14,000 r.p.m. for 20 min and the protein concentration in the supernatant was determined using the bicinchoninic acid assay (Pierce, Rockford, IL). Protein concentration for all samples was equalized to 5 mg ml–1 and the levels of the cytokine IL-13 and chemokines eotaxin and MIP-1α were determined using enzyme-linked immunosorbent assay.
Flow cytometric analysis of lung immune cell populations
PBS-perfused lungs were removed from euthanized mice. Lungs were finely cut and digested in DMEM (Invitrogen) with 50 U ml–1 collagenase type I (Invitrogen) and 13 μg ml–1 DNAse I (Roche) at 37 °C for 90 min. Samples were disrupted through a 70-μm cell strainer and subjected to red cell lysis. Cell numbers derived from each lung were calculated by Trypan blue staining and counting using a hematocytometer. Lung T cells were detected with anti-CD3 fluorescein isothiocyanate monoclonal antibody (BD Bioscience). Lung CD4+ T cells were detected with anti-CD4-phycoerythrin or fluorescein isothiocyanate monoclonal antibodies (GK1.5; BD Pharmingen, San Jose, CA), CD8+ T cells with anti-CD8-biotinylated monoclonal antibody (Ly2; BD Pharmingen), and B cells with anti-CD19-fluorescein isothiocyanate monoclonal antibody (BD Pharmingen). Eosinophils were detected by anti-CD11C-biotin (BD Pharmingen) and anti-GR-1-FITC (BD Pharmingen) monoclonal antibodies and identified as a CD11c-low, GR-1-intermediate population. Mast cells were detected by anti-CD117-APC and anti-FCɛR-phycoerythrin. The homogeneity of all populations was confirmed through cell sorting (FACSVantage; BD Biosciences) and microscopic confirmation following staining (not shown).
For PSMC-1 cells, 1 × 105 cells were plated in six-well plates and treated overnight with 10 μg ml–1 NES. After treatment the cells were detached by trypsinization and stained with anti-IL-4Rα-biotin and streptavidin-APC.
Preparation of NES products
N. brasiliensis L3 were harvested in 0.65% NaCl solution and resuspended in a final volume of 25 ml. Larvae were washed in distilled water containing 250 ng ml–1 Fungizone followed by 5 washes with PBS containing 1,000 U ml–1 penicillin (Invitrogen) and 1 mg ml–1 streptomycin (Invitrogen). Larvae were then incubated in DMEM (Gibco-BRL, Invitrogen) containing 1,000 U ml–1 penicillin, 1 mg ml–1 streptomycin, and 100 μg ml–1 gentamicin (Sigma) for 1 h at 37 °C and then re-suspended in DMEM plus 100 μg ml–1 penicillin and 100 μg/ml–1 streptomycin at 10,000 larvae per ml and incubated overnight at 37. The culture was then filtered with a 0.20-μm syringe filter (Lasec, Cape Town, South Africa) and concentrated using a 3,000 Da cutoff Amicon filter (Millipore, Cork, Ireland). Protein concentration was determined using the bicinchoninic acid assay (Pierce).
[3H]-thymidine cell proliferation assay
PSMC-1 cells were plated at a density of 3,000 cells per well in a 96-well plate (Nunc, Roskilde, Denmark) in 100 μl of DMEM supplemented with 10% fetal calf serum and incubated overnight at 37 °C. Cells were then treated with NES or recombinant cytokines IL-4 and/or IL-13 at varying concentrations followed by addition of 1.25 μCi of [3H]-labeled thymidine per well. Cells were then incubated for 24 h at 37 °C to enable radiolabeled [3H]-thymidine incorporation. Cells were harvested onto filter papers using a cell harvester (Insel, Hanble, UK) and dissolved in scintillation fluid (Zinsser Analytic, Berkshire, UK). The β radiation level was measured using a scintillation counter as disintegrations per min and is an indication of the amount of [3H]-thymidine incorporated.
Effect of NES on phorbol myristate acetate- induced proliferation of PSMC-1 cells
To determine the potential effects of NES on PKC activation, PSMC-1 cells were treated with the PKC activator phorbol myristate acetate (Sigma) in conjunction with NES and/or the PKC inhibitor bisindoylmalemide (Sigma). PSMC-1 cells were plated at 3,000 cells per well in a 96-well plate and incubated overnight at 37 °C. Cells were subsequently treated with either 10 μg ml–1 NES or control (PBS) immediately after addition of 100 nM phorbol myristate acetate. Changes in cellular proliferation were determined using [3H]-thymidine incorporation as described previously.
Propidium iodide cell cycle analysis
PSMC-1 cells were plated at 1 × 105 cells per well in a six-well plate (Nunc) and incubated at 37 °C overnight. Cells were then treated with 10 μg ml–1 NES or PBS and incubated for a further 24 h. Cells were then harvested and fixed with ice-cold 70% ethanol, treated with 100 ng ml–1 RNAse A and stained with 50 μg ml–1 of propidium iodide. DNA content of cells was then analyzed by flow cytometry using the ModFit program (Verity Software House, Topsham, ME).
Statistical analysis
Values are given as mean±s.d. Significance differences were determined using one-way analysis of variance and differences between groups were established using Tukey's post hoc test (Prism Software, http://www.prism-software.com).
References
Zhao, A. et al. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J. Immunol. 171, 948 (2003).
Zhao, A. et al. Contribution of 5-HT2A receptor in nematode infection-induced murine intestinal smooth muscle hypercontractility. Gastroenterology 131, 568 (2006).
Akiho, H., Blennerhassett, P., Deng, Y. & Collins., S.M. Role of IL-4, IL-13, and STAT6 in inflammation-induced hypercontractility of murine smooth muscle cells. Am. J. Physiol. Gastrointest. Liver Physiol. 282, G226 (2002).
Laporte, J.C. et al. Direct effects of interleukin-13 on signaling pathways for physiological responses in cultured human airway smooth muscle cells. Am. J. Respir. Crit. Care Med. 164, 141 (2001).
Brombacher, F. The role of interleukin-13 in infectious diseases and allergy. Bioessays 22, 646 (2000).
McKenzie, G.J., Bancroft, A., Grencis, R.K. & McKenzie, A.N. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Curr. Biol. 8, 339 (1998).
Barner, M., Mohrs, M., Brombacher, F. & Kopf, M. Differences between IL-4R alpha-deficient and IL-4-deficient mice reveal a role for IL-13 in the regulation of Th2 responses. Curr. Biol. 8, 669 (1998).
Takeda, K. et al. Essential role of Stat6 in IL-4 signalling. Nature 380, 627 (1996).
Grunig, G. et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 282, 2261 (1998).
Foster, P.S . STAT6: an intracellular target for the inhibition of allergic disease. Clin. Exp. Allergy 29, 12 (1999).
Urban, J.F. Jr . et al. IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity 8, 255 (1998).
Kuperman, D.A., Huang, X., Nguyenvu, L., Hölscher, C., Brombacher, F. & Erle, D.J. IL-4 receptor signaling in Clara cells is required for allergen-induced mucus production. J. Immunol. 175, 3746 (2005).
Radwanska, M. et al. Deletion of IL-4Ralpha on CD4 T cells renders BALB/c mice resistant to Leishmania major infection. PLoS Pathog. 3, e68 (2007).
Nieuwenhuizen, N., Herbert, D.R., Lopata, A.L. & Brombacher, F. CD4+ T cell-specific deletion of IL-4 receptor alpha prevents ovalbumin-induced anaphylaxis by an IFN-gamma-dependent mechanism. J. Immunol. 179, 2758 (2007).
Leeto, M., Herbert, D.R., Marillier, R., Schwegmann, A., Fick, L. & Brombacher, F. TH1-dominant granulomatous pathology does not inhibit fibrosis or cause lethality during murine schistosomiasis. Am. J. Pathol. 169, 1701 (2006).
Herbert, D.R. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity 20, 623 (2004).
Horsnell, W.G. et al. Delayed goblet cell hyperplasia, acetylcholine receptor expression, and worm expulsion in SMC-specific IL-4Ralpha-deficient mice. PLoS Pathog. 3, e1 (2007).
Marilier, R . et al. IL-4Rа-responsive smooth muscle cells increase intestinal hypercontractility and contribute to resistance during acute schistosomiasis. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G943–G951 (2010).
McNeil, K.S., Knox, D.P. & Proudfoot, L. Anti-inflammatory responses and oxidative stress in Nippostrongylus brasiliensis-induced pulmonary inflammation. Parasite Immunol. 24, 15 (2002).
Matsuda, S., Tani, Y., Yamada, M., Yoshimura, K. & Arizono, N. Type 2-biased expression of cytokine genes in lung granulomatous lesions induced by Nippostrongylus brasiliensis infection. Parasite Immunol. 23, 219 (2001).
Marsland, B.J., Kurrer, M., Reissmann, R., Harris, N.L. & Kopf, M. Nippostrongylus brasiliensis infection leads to the development of emphysema associated with the induction of alternatively activated macrophages. Eur. J. Immunol. 38, 479–488 (2008).
Horsnell, W.G. et al. Delayed goblet cell hyperplasia, acetylcholine receptor expression, and worm expulsion in SMC-specific IL-4Ralpha-deficient mice. PLoS Pathog. 3, e1 (2007).
Venkayya, R., Lam, M., Willkom, M., Grünig, G., Corry, D.B. & Erle, D.J. The Th2 lymphocyte products IL-4 and IL-13 rapidly induce airway hyperresponsiveness through direct effects on resident airway cells. Am. J. Respir. Cell Mol. Biol. 26, 202 (2002).
Cohn, L., Homer, R.J., MacLeod, H., Mohrs, M., Brombacher, F. & Bottomly, K. Th2-induced airway mucus production is dependent on IL-4Ralpha, but not on eosinophils. J. Immunol. 162, 6178 (1999).
Cruickshank, S.M. et al. Rapid dendritic cell mobilization to the large intestinal epithelium is associated with resistance to Trichuris muris infection. J. Immunol. 182, 3055 (2009).
Li, R.W., Sonstegard, T.S., Van Tassell, C.P. & Gasbarre, L.C. Local inflammation as a possible mechanism of resistance to gastrointestinal nematodes in Angus heifers. Vet. Parasitol. 145, 100 (2007).
Ierna, M.X., Scales, H.E., Saunders, K.L. & Lawrence, C.E. Mast cell production of IL-4 and TNF may be required for protective and pathological responses in gastrointestinal helminth infection. Mucosal Immunol. 1, 147 (2008).
Fabre, V. et al. Eosinophil deficiency compromises parasite survival in chronic nematode infection. J. Immunol. 182, 1577 (2009).
Stevenson, L.M., Huntley, J.F., Smith, W.D. & Jones, D.G. Local eosinophil- and mast cell-related responses in abomasal nematode infections of lambs. FEMS Immunol. Med. Microbiol. 8, 167 (1994).
Cadman, E.T. & Lawrence, R.A. Granulocytes: effector cells or immunomodulators in the immune response to helminth infection? Parasite Immunol. 32, 1 (2010).
Yang, M. et al. Interleukin-13 mediates airways hyperreactivity through the IL-4 receptor-alpha chain and STAT-6 independently of IL-5 and eotaxin. Am. J. Respir. Cell Mol. Biol. 25, 522 (2001).
Kirstein, F. et al. Expression of IL-4Rа on smooth muscle cells is not necessary for development of experimental allergic asthma. J. Allergy Clin. Immunol. (2010) [E-pub ahead of print 23 June 2010].
Cohn, L., Homer, R.J., Marinov, A., Rankin, J. & Bottomly, K. Induction of airway mucus production By T helper 2 (Th2) cells: a critical role for interleukin 4 in cell recruitment but not mucus production. J. Exp. Med. 186, 1737 (1997).
Mearns, H. et al. Interleukin-4-promoted T helper 2 responses enhance Nippostrongylus brasiliensis-induced pulmonary pathology. Infect. Immun. 76, 5535 (2008).
Voehringer, D., Shinkai, K. & Locksley, R.M. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity 20, 267 (2004).
Mohrs, M., Shinkai, K., Mohrs, K. & Locksley, R.M. . Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity 15, 303 (2001).
Lillard, J.W. Jr ., Singh, U.P., Boyaka, P.N., Singh, S., Taub, D.D. & McGhee, J.R . MIP-1alpha and MIP-1beta differentially mediate mucosal and systemic adaptive immunity. Blood 101, 807 (2003).
Rosbottom, A. et al. Chemokine and cytokine expression in murine intestinal epithelium following Nippostrongylus brasiliensis infection. Parasite Immunol. 24, 67 (2002).
Hogaboam, C.M., Snider, D.P. & Collins, S.M. Activation of T lymphocytes by syngeneic murine intestinal smooth muscle cells. Gastroenterology 110, 1456 (1996).
Hogaboam, C.M., Snider, D.P. & Collins, S.M. Cytokine modulation of T-lymphocyte activation by intestinal smooth muscle cells. Gastroenterology 112, 1986 (1997).
Hogaboam, C.M., Snider, D.P. & Collins, S.M. Neuromuscular regulation of T-cell activation. J. Neuroimmunol. 75, 123 (1997).
Veler, H. et al. Superantigen presentation by airway smooth muscle to CD4+ T lymphocytes elicits reciprocal proasthmatic changes in airway function. J Immunol 178, 3627 (2007).
Hakonarson, H., Kim, C., Whelan, R., Campbell, D. & Grunstein, M.M. Bi-directional activation between human airway smooth muscle cells and T lymphocytes: role in induction of altered airway responsiveness. J. Immunol. 166, 293 (2001).
Ammit, A.J. et al. Effect of IL-6 trans-signaling on the pro-remodeling phenotype of airway smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 292, L199 (2007).
Quante, T. et al. Corticosteroids reduce IL-6 in ASM cells via up-regulation of MKP-1. Am. J. Respir. Cell Mol. Biol. 39, 208 (2008).
Diehl, S. et al. Induction of NFATc2 expression by interleukin 6 promotes T helper type 2 differentiation. J. Exp. Med. 196, 39 (2002).
Diehl, S. & Rincón, M. The two faces of IL-6 on Th1/Th2 differentiation. Mol. Immunol.o 39, 531 (2002).
Harnett, W. & Harnett, M.M. Inhibition of murine B cell proliferation and down-regulation of protein kinase C levels by a phosphorylcholine-containing filarial excretory-secretory product. J. Immunol. 151, 4829 (1993).
Allen, J.E. & MacDonald, A.S. Profound suppression of cellular proliferation mediated by the secretions of nematodes. Parasite Immunol. 20, 241 (1998).
Hewitson, J.P., Grainger, J.R. & Maizels, R.M. Helminth immunoregulation: the role of parasite secreted proteins in modulating host immunity. Maizels,. Mol. Biochem. Parasitol. 167, 1 (2009).
Mohrs, M., Holscher, C. & Brombacher, F. Interleukin-4 receptor alpha-deficient BALB/c mice show an unimpaired T helper 2 polarization in response to Leishmania major infection. Infect. Immun. 68, 1773 (2000).
Dunn, A. & Keymer, A. Factors affecting the reliability of the McMaster technique. J. Helminthol. 60, 260 (1986).
Giacomin, P.R., Wang, H., Gordon, D.L., Botto, M. & Dent, L.A. Loss of complement activation and leukocyte adherence as Nippostrongylus brasiliensis develops within the murine host. Infect. Immun. 73, 7442 (2005).
Salm, S.N. et al. Transforming growth factor-beta is an autocrine mitogen for a novel androgen-responsive murine prostatic smooth muscle cell line, PSMC1. J. Cell Physiol. 185, 416 (2000).
Mohrs, M., Ledermann, B., Köhler, G., Dorfmüller, A., Gessner, A. & Brombacher, F. Differences between IL-4- and IL-4 receptor alpha-deficient mice in chronic leishmaniasis reveal a protective role for IL-13 receptor signaling. J. Immunol. 162, 7302 (1999).
Acknowledgements
We thank Lizette Fick, Wendy Green, Zanaria Abbas, and Reagon Peterson for their technical assistance and Natalie Nieuwenhuizen and Saeeda Bobat for critical reading of the manuscript. The project was funded by the Royal Society (UK), National Research Foundation (SA), and Medical Research Council (SA). B. Dewals is a postdoctoral researcher of the Fonds National de la Recherche Scientifique (FNRS), Belgium.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Rights and permissions
About this article
Cite this article
Horsnell, W., Vira, A., Kirstein, F. et al. IL-4Rα-responsive smooth muscle cells contribute to initiation of TH2 immunity and pulmonary pathology in Nippostrongylus brasiliensis infections. Mucosal Immunol 4, 83–92 (2011). https://doi.org/10.1038/mi.2010.46
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2010.46